On Tuesday, 28 November 2017, WHO (World Health Organisation) issued a stern warning (published in two reports) – at least 10% of medical products currently on the market and in use in low and middle-income countries around the world may not be manufactured to regulatory standard or can be classified as fake.
Effectively, what this means is that many individuals around the world are currently using medications for which they are not likely receiving much benefit. WHO’s statement comes as a warning for governments to look into the matter further as it pertains to their country and take appropriate action as soon as possible.
False and substandard medical products place users (i.e. the public) at increased risk of further health concerns and illness, as well as the possibility of death. The impact of counterfeit medical products in circulation extends into the financial space too, wasting the finances of both users (the public) and healthcare systems alike. For countries of low and middle-income, individuals affected are particularly vulnerable to such negative effects.
WHO’s statement does not come out of nowhere, however, and neither is it a panicked reaction…
For a handful of years now, WHO has been receiving reported cases which have indicated substandard products (i.e. products that are not manufactured with the quality specifics they were designed for, and or/ are not adequately regulated), and this has prompted some serious investigation.
A total of 1 500 reports have been submitted to WHO since 2013. Medications topping the list of substandard or falsified products are antibiotics and antimalarial medications – two health concerns already in the spotlight.
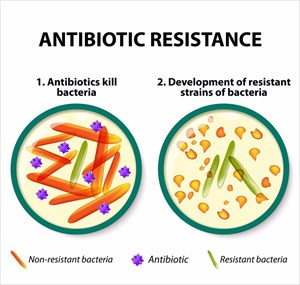
Why? Resistance has been noted to be rising to dangerously high levels across the globe. Health organisations are very much aware of emerging resistance mechanisms, which appear to be spreading throughout all corners of the globe. This has a direct impact on the ability of those in the medical field to treat a variety of illnesses, placing the public at serious risk.
Antibiotic resistance is making it increasingly difficult to gain the upper hand on common infectious diseases like tuberculosis, pneumonia, gonorrhoea, blood poisoning and foodborne illnesses, like salmonellosis. Treatment with antibiotics is becoming increasingly less effective.
One of the main reasons antibiotic resistance has become a global health issue is that many antibiotics are circulating the market without proper medical prescriptions. The public is also using antibiotics more often than they probably should as doctors in many countries are over-prescribing these drugs, operating in a manner that is not entirely compliant with standard treatment guidelines. This applies to both human and animal (veterinary) use of antibiotics.
WHO has strongly warned of a possible post-antibiotic era should these issues not be firmly controlled soon. Infections and minor health conditions which have to date been effectively controlled, may soon become life-threatening again. Not to mention the rising costs that will be placed on health sectors as the requirements for hospitalisations and related financial implications increase daily. The more first-line antibiotics become less effective, other, often more expensive, drugs are required for use, which directly impacts treatment and the duration of an illness (as well as costs health sectors and the public that much more).
Modern medicine achievements are also at risk – surgical procedures, chemotherapy and organ transplantations, to name a few, which rely on effective antibiotic treatment to keep infections at bay, could become increasingly dangerous for patients. Preventing infections is just as important as treating them effectively. It certainly does not help that false or substandard antibiotic products are circulating the market either.
Malaria too is another serious concern, and with substandard antimalarial drugs on the market, lights are flashing red here as well.
Significant strides have been made in getting malaria under better control. In recent years, much progress has been made. However, the World Malaria Report 2017 (1), also issued this month, has flagged a concern to take note of. While progress has been significant to date, it appears to have stalled somewhat.
An estimated 5 million additional cases of malaria were reported in 2016, in comparison with those in 2015. Malaria related deaths appeared to remain relatively the same between the two comparative years, and totalled around 445 000. Aside from global malaria targets for 2020 which aimed for a 40% reduction in reported cases and mortality rates now being at risk, health organisations around the world, and particularly those most vulnerable to the condition face a potential crisis – similar to issues surrounding fake antibiotic concerns, effective treatment is at risk of heading backwards if the associated challenges (one of which being substandard antimalarial medications) are not adequately overcome.
In the case of malaria, primary issues relate to insufficient funding for preventative measures (medications, insecticide-treated nets, sprays etc.). There is money floating around and being well utilised for its intended purpose, but not quite enough if targets are to be met. Major donations (we’re talking billions of dollars) have been made by leading economic countries like the USA, UK, France, Germany and Japan, and the impacts have been helpful (with many positive strides being made in the right direction), but still cases of malaria rose to 216 million (in 91 countries) from 211 million the previous year. The African continent carries the majority of the load when it comes to number of cases– a staggering 90%.
Where did all the reports for suspicious medical products come from?
In their statement, WHO indicated that 42% of the 1 500-odd reports received since 2013 have come from the African continent, 21% from the Americas, and 21% from Europe. The remainder of these reports have come from the Western Pacific (8%), Eastern Mediterranean (6%) and South-East Asia (2%).
WHO believes that the number of reports received may indeed total just a fraction of the broader problem, and suspects that many more cases have not been reported.
How has WHO taken action?
Reporting information relating to suspicious medical products is vital, but has not always been standard global practice. It has only really been since 2013 that global reporting of suspicious products has taken place. WHO took note of those being received and established the Global Surveillance and Monitoring System (GSMS) for the purpose of submitting reports and information on potentially falsified products. Since this has been implemented, more countries are actively reporting suspicious medical products, like medications, vaccines and medical devices.
For the past 4 years, the GSMS has allowed national regulatory authorities to access an interconnected network which can be used for the cross-referencing of reported suspicious medical products. The database is segmented by region and contains photograph libraries of products WHO has classified as substandard or falsified.
WHO is proactively encouraging health sectors around the world to take the matter seriously and not let suspicions slip under the radar. The implications are too serious, and as such, the organisation has trained a total of 550 regulators in 141 countries to ensure that they are equipped to respond to and tackle this issue. WHO has noted that the more trained regulators have become available to handle cases, the more reports have been coming in during the past few years – one of the main reasons why the organisation suspects many cases have been going unreported (until now).
WHO’s statement this week sets a serious tone, but also raises an alarm bell to take note of. Is the scale of the problem truly known at present? WHO does not appear to think so. What has come to light to date appears enough to alert the public and those in the medical field of the issue – hence the published statement and reports.
The first report WHO published this week details the data collected during the GSMS’s initial 4 years of operation, up until 30 June 2017. (2) The data covers substandard and falsified medical products (including medications, vaccines and in-vitro diagnostic tests) since July 2013 (around the time when the GSMS was established).
The second published report details studies on public health, as well as the socio-economic impact of substandard products. Studies were conducted by WHO and the Member State Mechanism. (3)
Since the initial reports of suspicious products from the GSMS, studies (as detailed in the second report) looked at more than 100 research papers (literary reviews). These papers have all been published detailing medicine quality surveys in 88 low and middle-income regions in the world, covering a total of 48 000 medicine samples. The report also looked at 178 sample reports from high-income countries, and thus the concluded estimates have been focussed on low to middle-income regions.
Two peer-reviewed models have also been included in the second report with data compiled by the University of Edinburgh and The London School of Hygiene and Tropical Medicine.
These research exercises help the organisation and healthcare providers to better understand the scale of the problem with the information at hand. The University of Edinburgh estimated that at least 72 000 of 169 000 annual paediatric cases of pneumonia may possibly be linked to infection related deaths as a result of poorly manufactured or falsified antibiotics.
The other exercise model done by the London School of Hygiene and Tropical Medicine estimated 116 000 more fatalities linked to malaria due to substandard antimalarial drugs in sub-Saharan Africa.
The WHO’s two reports present the most comprehensive recorded data of falsified and substandard medical products to date. WHO has noted that these research papers are merely the tip of the iceberg, meaning that there appears to be a lack of accurate data (i.e. much is still unknown), and the conclusions which they have drawn are only an estimated indication of the scale of the problem. The reports do provide a clear enough indication of what needs further understanding with regards to public health and the socio-economic impacts of such products circulating the market.
Along with antibiotics and antimalarial drugs, medical products for cancer treatment, heart related conditions, diabetes, mental health management, fertility and contraception (birth control) have also been flagged as suspicious – in both generic and patented products. WHO has also received suspicious reports regarding vaccines for meningitis and yellow fever. These findings are enough to be worrying, but more research is required.
From these papers and reported cases to the GSMS, an estimated 10.5% of suspicious medical products (currently in use in low to middle-income countries) have been deemed ‘substandard or false’ – meaning that they have failed to comply with standard guidelines and are thus ineffective for medical use.
Initially, reported cases of medical products to the GSMS (between 2013 and 2016) were categorised as:
- ‘Falsified’
- ‘Suspected falsified’
- ‘Substandard’
- ‘Diverted’
- ‘Stolen’
- ‘Unlicensed’
Following the World Health Assembly in 2016, these categories were whittled down to just ‘substandard’ and ‘falsified’. All reported cases have thus been categorised as one of the two.
Why are substandard / fake medical products doing the rounds?
In WHO’s statement, it is indicated that the problem is linked to limited supply and distribution of medical products in low and middle-income areas. It is far easier to access and use substandard products where quality control in the areas of manufacturing, supply and distribution are lacking.
Where a standard of quality is not enforced, and inadequate governance and regulation is lacking, WHO found the most reported cases, and especially so if those involved in medical product handling (i.e. wholesalers, distributors and retailers, as well as healthcare providers / employees) engage in unethical practices.
The online space also comes into play here. The advent of online pharmacies has also seen a relaxation of regulation when it comes to purchasing medical products. The scope of this influence requires more research as it has been noted that high-income countries favour this purchasing model more.
Demand for medications is fairly high with sales totalling in the trillions across the world. Demand growth has been most notable in middle-income countries too.
The hands of the regulatory powers have seemingly lost their grip. Adding to the problem is the manufacturing process. Those in the business of producing false products often manufacture and print packaging in separate countries. Components are regularly shipped to another location for assembly and distribution. The production of substandard medical products thus, happens in multiple places, making regulation that much more complex. Placing the cherry on top is that bank accounts facilitating the production are sometimes held offshore too and as such cannot be examined or sequestered by the regulatory bodies within certain countries.
So, what now?
Substandard and falsified medical products run a worrying risk of promoting antimicrobial resistance (bacterial, viral and some parasites). This means that public health, on a global scale, is at risk of mutant infections being transmitted across the world – predominantly through ease of cross-border / international travel, for instance. Once such organisms become resistant to current first-line medication treatments, they become increasingly difficult to eradicate and treat medically.
With medical products being substandard, this effectively means that drugs originally designed to destroy such pathogens and organisms at very specific doses, are rendered ineffective. The substandard products (or false products) have been found to either contain a fraction of the required dosage or are poorly manufactured, making the medication ineffective as the active ingredients are not properly released when used.
This means that the medication being used is simply not capable of being effective enough for treatment or preventative purposes. It may work to destroy some pathogens, but not all. This could mean that illnesses take longer to treat, or newer illnesses develop as pathogens adapt to better survive. It’s almost like going to war with only half of the army of your opponent.
Pathogens (infectious agents) which survive treatment mutate and become capable of resisting the lower dosage medical products (i.e. substandard / false products). More susceptible strains can effectively get the better of weaker medications, which gives more power to the opposing side, making illness more easily transmitted from person to person.
This also affects “the good guys”. Medication (generic and patented) that is manufactured to regulation and meets quality standards is also at risk of becoming ineffective in the fight against these new generation mutated illnesses, forcing medical advancements, which accomplished so much to date, back to the starting block. Financial losses and reputations of legitimate products are also placed at risk.
The estimated 10.5% figure regarding fake products issued as a warning is the WHO’s way of initiating awareness of a problem that needs to be nipped in the bud, and quickly. Essentially these categorised fake / substandard products are not approved by any above board, legitimate regulators, and those studied have been found to deliberately misrepresent ingredients and quality standards.
The Assistant Director-General for Access to Medicines, Vaccines and Pharmaceuticals at WHO, Dr Mariângela Simão has this to say with regards to countries taking heed to the organisation’s warning, “Countries need to assess the extent of the problem at home and cooperate regionally and globally to prevent the traffic of these products and improve detection and response.”
Effectively substandard and falsified medical products is a global concern, whether low, middle or high-income, and thus needs to be addressed in some way by all going forward. Healthcare is in our own hands.
References:
1. World Health Organisation. 29 November 2017. World Malaria Report 2017: http://www.who.int/malaria/publications/world-malaria-report-2017/en/ [Accessed 29.11.2017]
2. World Health Organisation. November 2017.WHO Global Surveillance and Monitoring System for substandard and falsified medical products: http://www.who.int/medicines/regulation/ssffc/publications/gsms-report-sf/en/ [Accessed 29.11.2017]
3. World Health Organisation. November 2017.A study on the public health and socioeconomic impact of substandard and falsified medical products: http://www.who.int/medicines/regulation/ssffc/publications/se-study-sf/en/ [Accessed 29.11.2017]