'Your daily cup of coffee can help to prevent cancer' – you smile at the headline as sip your morning cup, secure in the knowledge that you’re doing something great for your health, and even whilst sitting on your behind - it sure does beat going to the gym!
A month later however, as you once again sip your morning coffee, the headlines scream 'Chemicals in coffee cause cancer' and you nearly spray your last sip over the screen. 'How can this be?' you wonder nervously as you click to find out more. You scan the opening lines ‘New research shows…’ and with that you close the article, as you consider your options, head to the kitchen to pour out the remainder of your now defunct morning ritual or finish the cup.
Can you trust what you read when it’s all so contradictory?
Medical and health news can be confusing and more often than not, seemingly contradictory. This can be downright frustrating when you’re trying to do the best you can for your health. Not to mention that seeing continually conflicting articles fosters the perception that no one in the medical field knows anything for sure. It's ‘all just guesswork’, right?
Wrong. The problem is not the medical field, but rather in the way scientific findings are sensationalised and misinterpreted by certain individuals and the media, crafted to be used as click bait and then shared on social media.
Of course, medicine is continually evolving and when you take into account the complexity of the human body, and how much we’re still learning about it, new findings may, in fact, contradict older ones. However, for newer findings to replace generally accepted, proven ones, extensive trials of a certain nature are required (more on that later). It’s not an overnight process that has medical professionals scrambling back and forth based on every new research finding produced. Medicine is a science that involves sound methodology and regulation.
The cause for concern arises due the fact that while something as insignificant as whether or not you drink a cup of coffee may not have any real impact on your long term health, buying into other medical and health related headlines may be hazardous.
This is especially true if what you read in the media leaves you questioning or abandoning your medically prescribed treatment for a specific health condition in favour of the latest recommendations or due to findings or warnings reported on by the media. In fact, this is one of the ways in which the anti-vax movement got its start1, and also why thousands of people prescribed statins for high cholesterol have stopped taking them2,3, putting many at a higher risk of experiencing a significant cardiovascular event such as a heart attack or stroke. The list goes on and on.
So, what is one to do?
There are a few things to do and questions to ask yourself when evaluating whether what you’re reading is actually worth your time and consideration or not.
Is what you’re reading supported by scientific research?
If the piece that you’re reading is about a treatment or lifestyle choice that supposedly prevents or causes a specific disease, but doesn’t make reference to hard scientific evidence, then treat it with a pinch of salt. The same goes for findings that are yet to be published.
Conference abstracts are another area where caution should be exercised as research presented at conferences is often in the early stages and hasn’t been subject to expert review. Another shortcoming of conference abstracts is that they rarely provide in-depth detail on the particular study’s methodology, which makes judging the level of research conducted a challenge.
Therefore, if an article states that findings presented at a conference show that you should stop or start doing something specific, or that a certain treatment is better than another, don’t take this too seriously. Also, please don’t panic and make an appointment with your GP as you’ll just be wasting your money.
These should, however, not be your only evaluation criteria for evaluating information on which to base your health and medical decisions. There are other important factors to take into consideration. For instance…
What type of study or trial was conducted, how many subjects were involved and most importantly, were they human?
You’ll often read a headline about a breakthrough in treating a certain disease or ‘miracle cure’, only to find that it has been tested on mice or some other lab animal. Oftentimes, these stories feature images of people or how the ‘cure’ would theoretically work in humans which can be misleading.
Animal studies are important. However, humans are not mice or any other animal for that matter. Often drugs and treatments that show promise in a laboratory setting that involves animals don’t work in humans.
It is also important to keep in mind that while something shows promising results in animals, even if it does do the same in humans, it’s often a long way from being available to the public. So realistically a cancer cure will probably not be available within one year of a study showing promising results in animal trials and a wonder food that ‘cures’ lab mice of Crohn’s disease is not a reason to start eating it too, or not yet anyway, despite what the headlines say. Likewise, if a study involves humans but in small numbers, the results have less statistical significance than a study conducted on larger groups and you should be prepared to wait for more information and be mindful of the fact that you may have to wait a very long time.
It takes approximately 12 years for a drug to make it from the lab to the pharmacy shelf. That is, if it makes it at all. Research shows that just 32% of drugs trialled make it to phase III trials, and only one in 10 make it to market4.
Here’s how the various trial stages work and what that means5:
- Pre-clinical non-animal tests: These types of studies are conducted using a variety of research tools including computer modelling, cell cultures (in vitro), automatic screening, microbial studies and more. This eliminates all potentially toxic and obvious non-viable drugs and treatments before they go on to the more expensive animal testing phase.
- Pre-clinical animal testing (in vivo): Drugs/treatments and dosages are then tested on animals in order to determine the potential therapeutic value of the proposed drug/treatment and more importantly the safety of trialling it in humans. Once this is established, human trials may begin.
- Phase 0 trials: After passing pre-clinical animal trials, some drugs or treatments go into phase 0 clinical trials, others skip this stage and go to phase 1 testing. Phase 0 or ‘micro-dosing studies’ involve small groups of participants (10 – 15) who receive dosages of the drug being tested at levels below that which is considered to be therapeutic (i.e. these dosages are not large enough to treat the condition they were designed for). This phase is used to ascertain whether or not the drug behaves the same way in humans as it does in animals and in the expected manner.
- Phase I clinical trials: These trials are used to determine the safety and tolerability of the drug/treatment being tested. Participant are usually healthy individuals unless there are indications that the drug won’t work in them and requires individuals to have the health condition or disease in question. Participant groups usually range from 20 to 100 individuals. Dosages are tested, starting low and increasing while the effects are monitored.
- Phase II clinical trials: If the drug / treatment being tested in phase one is well tolerated, trials progress to phase two. In this phase the efficacy of the drug is tested, and appropriate therapeutic doses are determined. The number of participants increases dramatically. Side effects and safety observations continue.
- Phase III: Phase III trials focus on efficacy and take place over an extended period, involve larger numbers of participants than the previous phases and occur under conditions that reflect clinical life. Trials of a higher quality divide participants into two randomised groups. One group is given the medication or treatment while the other is given the placebo or traditional treatment for the condition in question (this group is referred to as the ‘control group’). This stage may also be divided into two separate phases – IIIA and IIIB. Applications for regulatory approval are made after phase IIIA is completed and phase IIIB is conducted to obtain further safety data and evidence while awaiting approval.
- Phase IV: Once approval from a national regulatory authority is obtained, some drugs and treatments continue clinical trials. These are referred to as post-marketing surveillance studies and allow researchers to obtain added information regarding side effects and safety as well as determine longer term risks and benefits. For certain drugs and treatments these types of trials are required.
Does the article mention a control group?
Research is designed to answer many questions and there are different types of studies to do that. If an article mentions anything about a treatment or the effect of a behaviour or exposure to something, then there needs to be a study and that study must have involved a control group.
A control group essentially allows researchers to compare what happens when a certain treatment is administered, behaviour is present or exposure to something occurs in comparison to when it doesn’t.
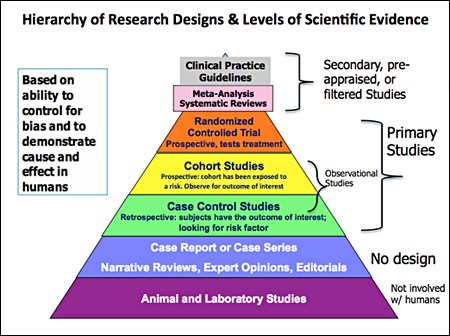
So, when you’re looking at an article, it should mention a Randomised Controlled Trial (RCT) – these are the ‘gold standard’ of trials. If you see any other type of study mentioned, maintain a healthy degree of scepticism about what you’re reading.
Here’s a graphical representation of the types of studies that may be used so you know what you’re looking at:
Check what the headline says versus what the study really involved
This may seem obvious, but sometimes journalists extrapolate from the research content to produce a snappy headline that is sure to get clicks.
For example, if a headline reads ‘Diet drinks may give you a stroke’, you need to be sure that the study actually looked at the instances of strokes and not just a rise in blood pressure (unmanaged high blood pressure can lead to strokes6).
Also look at the population group studied, and if the research accounted for other factors that may also increase the risk of either elevated blood pressure or stroke (for example, age, gender and lifestyle factors).
Sometimes extrapolations may prove to be true, but this is not always the case. If the focus is on the outcome and not the research, exercise caution in what you believe and the adjustments you make accordingly.
Who funded and conducted the study?
Many trials are funded by manufacturers and conducted by their employees. This can lead to all kinds of conscious and sub-conscious bias. While this isn’t a reason to discount a trial, as many sponsored ones are sound and produce solid data, keep the potential for conflict of interest in mind and think critically about what you’re reading.
Who’s to blame?
We can’t always blame journalists or the media for misleading information and headlines, sometimes that comes in with editing, other times the researchers or marketers of certain drugs, treatments of products over-extrapolate and make unfounded claims that the evidence just doesn’t support in statements and press releases. These are then picked up by journalists who work off the information they’ve been given.
In a world of information overload, fake news, misinterpretation and click bait, the onus is on you to do some very critical thinking and not believe everything you read, as it could really affect your health.
References
1. Hussain, A., Ali, S., Ahmed, M. and Hussain, S. (2018). The Anti-vaccination Movement: A Regression in Modern Medicine. Cureus, [online] 10(7). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6122668/ [Accessed 22 Feb. 2019].
2. Kriegbaum, M., Liisberg, K. and Wallach-Kildemoes, H. (2017). Pattern of statin use changes following media coverage of its side effects. Patient Preference and Adherence, [online] Volume 11, pp.1151-1157. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5513880/ [Accessed 22 Feb. 2019]
3. Bazian (2016). Heart attacks linked to media statin reports ... reports media. [online] nhs.uk. Available at: https://www.nhs.uk/news/heart-and-lungs/heart-attacks-linked-to-media-statin-reports-reports-media/ [Accessed 22 Feb. 2019].
4. Huss, R. (2016). The High Price Of Failed Clinical Trials Time To Rethink The Model. [online] Clinicalleader.com. Available at: https://www.clinicalleader.com/doc/the-high-price-of-failed-clinical-trials-time-to-rethink-the-model-0001 [Accessed 22 Feb. 2019].
5. Understanding Animal Research. (2013). Nine out of ten statistics are taken out of context | Understanding Animal Research | Understanding Animal Research. [online] Available at: http://www.understandinganimalresearch.org.uk/news/communications-media/nine-out-of-ten-statistics-are-taken-out-of-context/ [Accessed 22 Feb. 2019].
6. heart.org. (2016). How High Blood Pressure Can Lead to Stroke. [online] Available at:https://www.heart.org/en/health-topics/high-blood-pressure/health-threats-from-high-blood-pressure/how-high-blood-pressure-can-lead-to-stroke [Accessed 22 Feb. 2019].