On Tuesday, 5 December 2017, global healthcare company, Novo Nordisk (with headquarters in Denmark), announced the FDA (Food and Drug Administration) approval of their proposed injectable medication, Ozempic®, for the treatment of type 2 diabetes.
With more than 90 years’ experience developing innovative care for the treatment of the condition, Novo Nordisk operates in 77 countries around the world and markets their products in more than 165. The company employs more than 41 000 and has also achieved successful progress in treatments for growth disorders, haemophilia and obesity.
Ozempic® can be used, once a week, in conjunction with a healthy lifestyle comprised of a balanced diet and reasonable amount of activity. This GLP-1 RA (glucagon-like peptide-1 receptor agonist) treatment works to improve glycaemic control in adults.
Understanding the basics - type 2 diabetes
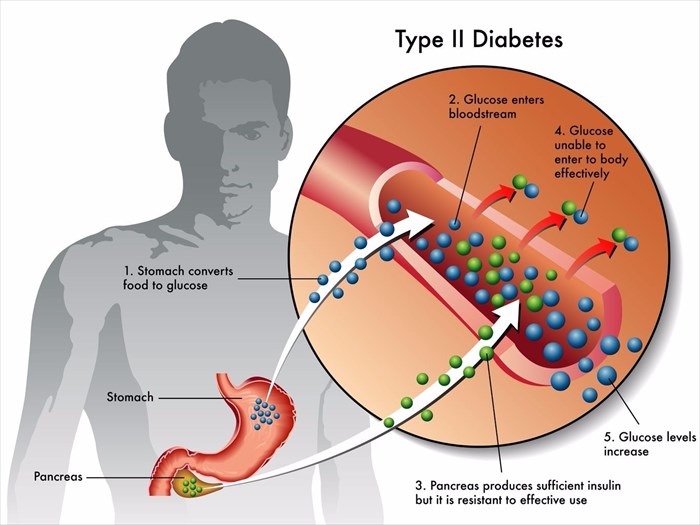
Characterised by a group of metabolic conditions, which influence how the body processes insulin (a hormone produced in the pancreas) and glucose (sugar), diabetes requires ongoing (daily) treatment in order for it to be successfully controlled and managed.
Type 2 diabetes most commonly develops with age (also known as adult-onset diabetes or non-insulin dependent diabetes - i.e. insulin resistance). In a nutshell, the body develops a resistance to the hormone and is incapable of using the substance effectively.
Glucose is a necessary energy source for the cells of the body, and is essential for healthy tissues and muscles, as well as brain function. Excess glucose in the system is typical of those living with diabetes (diabetes mellitus), and can lead to serious health complications. Insulin is normally secreted by the pancreas in order to assist with using and storing glucose (and carbohydrates and fat from the food we consume). An inadequate amount of insulin being produced is a critical factor in the development of diabetes.
If glucose is not sufficiently absorbed by the body’s cells, a build-up of the substance occurs in the bloodstream. Liver, fat and muscle cells are thus unable to store or use glucose efficiently and the resulting accumulation begins to damage the tiny blood vessels of the nervous system, and organs such as the heart, kidneys and eyes. This kind of impaired body function can place a person at risk of hyperglycaemia (high blood sugar levels).
Symptoms of the condition include excessive thirst and hunger, fatigue and a lack of energy, increased urination, blurry vision, dry mouth, itchy skin, slow-healing sores and cuts, and frequent yeast infections too.
With more and more youngsters becoming overweight at an early age, occurrences of type 2 diabetes have risen alarmingly, especially if individuals lead unhealthy lifestyles characterised by poor nutrition and inactive or sedentary living.
There is no cure for diabetes (type 1 or type 2), and if ineffectively treated, a person is at risk of complications such as kidney failure or kidney disease, nerve tissue damage, stroke, heart disease and blindness.
Current treatment of the condition involves regular monitoring of blood sugar levels (several times a week or even 4 to 8 times a day, depending on a person’s condition) with a glucose monitor, and or / an A1C testing every 2 to 3 months at the doctor’s office, basal insulin therapy (which controls blood sugar levels between meals and when at rest / sleep), oral-glucose lowering therapy and other oral medications to help stimulate insulin production and secretion, as well as suppress the production and secretion of glucose from the liver.
Novo Nordisk hopes to introduce Ozempic® in the mix of treatment options to effectively keep the condition under better control for adults. And it comes at a very appropriate time…
Diabetes, a global health issue
A chronic condition, diabetes is estimated to affect around 8.8% of the world’s population at present. For a number of years now, this medical condition has been on the global public health agenda as new diagnoses have been steadily climbing, particularly in low and middle-income regions / countries.
In 2011, the condition was recognised, at a WHO General Assembly, as one of four priority NCDs (non-communicable diseases) requiring targeted commitment by countries and their health sectors. This is because the condition can result in disability (due to blindness, kidney failure, stroke, heart attack and lower limb amputation, which are among the most common complications) and premature death. All are potentially preventable, making treatment of the disease a significant priority. Participating nations signed a Political Declaration on the Prevention and Control of NCDs. (1)
The World Health Organisation (WHO) shone another spotlight on diabetes on World Health Day (an annual awareness day on 7 April) in 2016, calling for a more proactive approach to the treatment of the disease, as well as implement more effective prevention tools. The first WHO Global report on diabetes was issued the same year. (2)
Following the declaration, a comprehensive global framework was developed in 2013. The approach laid out interventions which were multi-sectoral and population-wide, incorporating cost-effective and affordable, as well as evidence-based strategies for the achievement of 9 voluntary global targets, set to be reached by 2025.
In 2015, the United Nations General Assembly adopted a similar agenda with targets marked for the year 2030. Participating countries signed on the dotted line to reduce premature fatalities by at least one third, provide access to affordable medical treatment (namely, insulin, oral hypoglycaemic medications, as well as those to control blood pressure and lipids) and achieve universal health coverage (i.e. reduce climbing figures related to obesity and diabetes, promote physical activity and healthy lifestyle and behaviours, as well as lower exposure risks to tobacco). Successfully done, this strategy is aimed at achieving considerably lower figures for diabetes and associated medical complications. If successful, it could also result in a significant reduction in the number of type 2 diabetes occurrences.
Why the focus on diabetes and healthy overall living?
In the WHO global report, it was shown that the number of diagnosed cases was on the rise, virtually doubling between 1980 (4.7%) and 2014 (8.5%). This brought the total of known condition cases to 422 million adults, compared to 108 million calculated in 1980. The sharpest rise in cases appeared to be type 2 diabetes, which also had a strong association with rising numbers in overweight and obese individuals. Unhealthy weight gain and obesity are considered preventable, and thus a large portion of individuals being diagnosed with diabetes as adults should not technically be suffering from the illness. Due to the fact that weight management can prevent obesity, the condition should not be high on the list of risk factors for diabetes.
The rising figures were also noted to have contributed to around 1.5 million fatalities associated with diabetes in 2012 alone (many before the age of 70). (3) Death percentages could all be attributed to higher than normal blood glucose levels associated with diabetes, and particularly in countries of low to middle income (which previously did not see the majority of diagnosed cases). The report also highlighted an increased number of diagnoses amongst children (along with notable increases in poor weight management amongst minors leading to increasing number of children being overweight or obese), as well as adults.
Hence, the report was angled at encouraging governments and healthcare sectors around the world to promote healthier life choices among the public, as well as treat diagnosed conditions more effectively.
On the back of the 2011 Political Declaration on the Prevention and Control of NCDs, which recognised the spike in diagnoses and the implications of the condition, nutrition (balanced diet) and a healthy level of physical activity were once again highlighted as much a priority as effective treatment measures.
The bottom line is to achieve fundamental goals in better controlling diabetes as a condition and implementing preventative measures which contribute to fewer complications and risk factors.
Incoming Ozempic®
The active ingredient in this new medication, developed by Novo Nordisk, is semaglutide, a GLP-1 (human glucagon-like peptide) receptor agonist which can be administered by injection. FDA approval of this product comes on the back of satisfactory results from a SUSTAIN clinical trial programme (a global clinical programme which comprises of 8 x phase-3 clinical trials) (4), as well as a positive recommendation given on the 18th of October 2017 by the FDA Advisory Committee. Trials have been conducted on several thousand male and female adults (of various ages, length of time living with the condition and ethnic groups) with type 2 diabetes thus far, looking at safety and efficacy of the product. (5)
Overall results of the trial showed that Ozempic® has a unique clinical profile, displaying significantly lower levels of HbA (glycosylated haemoglobin) in comparison with type 2 diabetes participants using placebos, sitagliptin, exenatide extended-release and insulin glargine U100.
Trial results also appear to have had an effect on the body weight of participants, showing significant reductions. Reduction results detailed the use of the medication in conjunction with heathy eating practices and regular exercise. With obesity figures associated with type 2 diabetes on the rise, this is an added benefit that is in line with global targets, which could help health sectors reach these objectives.
Some of the participants had high cardiovascular risk profiles, and others renal (kidney) disease. Some participants already had diagnosed kidney disease.
Trials were extensive and overall, the medication appeared to demonstrate a well-tolerated profile by participants, making it considerably safe to use. Mild to moderate nausea seemed to be the most common complaint when it came to side-effects, but this did appear to subside after a short period of time. Other adverse effects included a little vomiting, diarrhoea, constipation and abdominal pain. Less common were instances of pancreatitis, thyroid tumours, vision changes, allergic reactions and kidney failure.
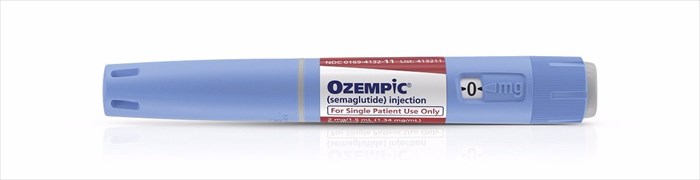
Approval by the FDA allows for this prescription medication to be used in two dosages - 0.5mg and 1 mg – from a pre-filled device, the Ozempic® Pen. Each pre-filled pen can be used to delivers doses of 1mg.
This clear and colourless medication solution is designed as a once-weekly analogue of GLP-1 with significant potential in treating the disease, but is not yet recommended as a first-line choice of therapy for improving glycaemic control in adults. It is however recognised as an effective treatment option for helping to regulate insulin in diabetics whose bodies are either not able to produce sufficient amounts naturally, or are incapable of using the hormone effectively enough.
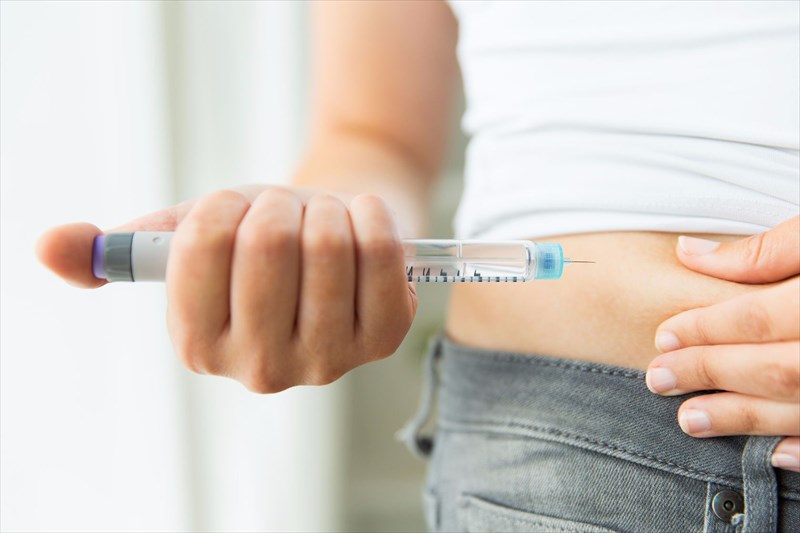
Using the Ozempic® Pen, patients can inject the medication subcutaneously (just beneath the skin) in the abdomen, upper arm or thigh, avoiding muscles and veins. The medication is thus not to be administered intramuscularly or intravenously.
Ozempic® is recommended for use once a week at a dose of 0.5mg, on the same day, at any time during the chosen day (with or without meals). Treatment is recommended to commence at a lower dosage, however, for the initial 4 weeks (0.25mg once a week). This dosage is intended as an initiation to treatment and not for effective glycaemic control. After 4 weeks, the dosage can be safely increased to 0.5mg once a week and then 1mg after another month period if needed.
The active ingredient stimulates insulin secretion, inhibits glucagon secretion, and thereby reduces (balances) blood glucose levels in the body. A minor delay in gastric emptying also helps to lower blood glucose levels soon after a big meal is consumed (known as the postprandial phase when blood sugar is typically higher).
Going forward, Novo Nordisk will be conducting a paediatric trial for participants under the age of 18 (aged 10 to 17). The study will be a 26-week randomised, double-blind placebo-controlled trial, which is the gold standard of clinical trials, in order to assess safety and efficacy for this age group.
The FDA recognised in their letter of approval that clinical trials with participants aged between 0 and 9 are not possible at present as too few children in this age range can be studied for the time being. (6)
FDA approval comes with another requirement, requesting that the organisation add the medication to the MTC (medullary thyroid carcinoma) registry, which consists of other long-acting GLP-1 therapeutic products.
A day after receiving FDA approval, Novo Nordisk held a conference call (on 6 December 2017) with potential medication investors.
Executive Vice President and Chief Science Officer at Novo Nordisk, Mads Krogsgaard Thomsen expressed excitement for the first FDA approval of the product, and said that the organisation is looking forward to “making this important innovation available to people in the US with type 2 diabetes in the beginning of 2018.” She also went on to say that, “We believe it has the potential to set a new standard for the treatment of the disease.”
Ozempic® is now under review by numerous regulatory agencies, which include the Japanese Pharmaceuticals and Medical Devices Agency, and the European Medicines Agency.
Considerations for use
With the medication having now been given the greenlight for use in the USA early next year, the approval comes with a fair amount of care recommendations.
For now, the medication is only to be prescribed to adults with type 2 diabetes, but not as a substitute for other treatment including insulin therapy. The medication is also not recommended for use by type 1 diabetes patients or those with diabetic ketoacidosis (a life-threatening complication of diabetes, whereby the body’s cells are unable to receive glucose, and instead the substance accumulates in the blood).
The medication is recommended to be prescribed as an injectable prescription therapy, along with nutritious diet choices and adequate levels of exercise activity.
Some use considerations and limitations of the medication include:
- Avoiding use of this medication if patients already have / have had pancreatitis (inflammation of the pancreas, or even the kidneys), a history of diabetic retinopathy, have had or have a family member diagnosed with MTC (medullary thyroid carcinoma) or an endocrine condition like Multiple Endocrine Neoplasia syndrome type 2 (MEN-2), have a known allergy or hypersensitivity to semuglutide (or any of the other non-active ingredients - propylene glycol, disodium phosphate dihydrate, phenol and water), or are younger than 18 years of age.
- Women who are pregnant or plan to become pregnant should also take precautions when considering this medication. The product is not recommended to be used at least 2 months prior to falling pregnant (due to a long washout period of semaglutide), nor during pregnancy. Other treatment options for controlling blood sugar will need to be prescribed.
- It is also not recommended that breastfeeding mothers use this medication, as it has not been determined if it is possible for the drug to be passed to a baby through breastmilk (i.e. this has not been studied in human trials. There is, however, some limited data accumulated from animal studies).
- A treating physician will take into consideration medication interactions whereby certain prescription and over-the-counter drugs may influence the effectiveness of the medication when taken at the same time. All vitamins and herbal supplements must also be disclosed to a physician before starting treatment as this can have an effect too. For instance, the risk of low blood sugar levels may increase when the medication is used at the same time as others containing insulin or sulfonylurea. Insulin can be taken at the same time (but must be monitored carefully), and must not be mixed with Ozempic® and administered together. The two can be administered in the same bodily area, but not injected adjacent to one another. In general, this medication may delay the processes of gastric emptying (which has a role in regulating blood sugar levels) and will have a direct impact on the absorption capabilities of other medications and supplements.
- Treatment with this medication should be discontinued (or re-evaluated) if inflammation of the pancreas develops (normally accompanied by persistent pain in the abdomen, with or without vomiting), or signs of kidney problems (like nausea, vomiting, diarrhoea and dehydration), as well as allergic reactions (breathing difficulties, skin rashes and itching) or vision changes.
Dosages of Ozempic® may require adjustments during treatment if levels of exercise activity or diet habits change, weight is either gained or lost, stress levels increase, other illness develops, including infections, or surgery is required.
References:
1. World Health Organisation. 24 January 2012. United Nations General Assembly: http://www.who.int/nmh/events/un_ncd_summit2011/political_declaration_en.pdf [Accessed 08.12.2017]
2. World Health Organisation. 2016. Global Report on Diabetes: http://www.who.int/diabetes/global-report/en/ [Accessed 08.12.2017]
3. World Health Organisation. November 2017. Diabetes Fact Sheet: http://www.who.int/mediacentre/factsheets/fs312/en/ [Accessed 08.12.2017]
4. ClinicalTrials.gov. 28 August 2013. Efficacy and Safety of Semaglutide Once-weekly Versus Sitagliptin Once-daily as add-on to Metformin and/or TZD in Subjects With Type 2 Diabetes (SUSTAIN™ 2): https://clinicaltrials.gov/ct2/show/NCT01930188 [Accessed 08.12.2017]
5. FDA.gov. OZEMPIC (semaglutide) injection, for subcutaneous use) label: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf [Accessed 08.12.2017]
6. FDA.gov. NDA approval letter: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/209637s000ltr.pdf [Accessed 08.12.2017]