The mere thought that you could develop a progressive and irreversible disorder of the brain, like that of Alzheimer’s disease, may fill you with some degree of fear.
When you’ve led a self-sufficient lifestyle throughout your adult years, any progressive deterioration in the ability to think clearly, remember details and function accordingly during older age is unnerving. No one wishes to develop such a condition, nor see a loved one through it…
As yet, there is no cure for Alzheimer’s disease on the horizon, and as such, finding one is part of ongoing research. Every so often, updated study findings surface, and medical researchers and professionals are able to learn a little more about this complex medical condition. Clues, ideas and potential influences all filter through a significantly funded research process. The goal is to better understand the highly intricate nature of this disease so as to possibly diagnose it sooner and develop more effective treatment processes.
The condition is characterised by many complex changes in the brain, but scientists do not yet have a clear understanding of the direct causes of the disease. The development of Alzheimer’s is largely attributed to the accumulation of amyloid proteins and sticky plaques in the brain which contribute to the deterioration of brain cells that influences the progressive decline of cognitive function. There is still a great deal to learn about the disease, including its causes and associated triggers if any further medical advancements are to be made.
The latest study, published in the journal, Neuron on 21 June 2018, highlights a potential connection between the development of Alzheimer’s disease and certain strains of the human herpesvirus – the HHV-6A and HHV-7 strains to be more precise. Through their analysis, researchers noted that the brain tissues of individuals with diagnosed Alzheimer’s disease appeared to have levels of these strains that were considerably higher compared to those without the condition. (1)
The team consisted of researchers from the Arizona State University’s Banner Neurodegenerative Disease Research Center, as well as the Icahn School of Medicine at Mount Sinai (New York) in the USA. Their findings detail an interesting association which will surely invite further study down the line.
For now, what could this study potentially mean for those with the condition?
As a form of dementia, Alzheimer’s is estimated to affect between 60% and 70% of individuals diagnosed with this type of condition around the world. Approximately 50 million people are believed to be living with some form of dementia across the globe. (2) With such a high prevalence, it’s not surprising that brain disorders, like dementia / Alzheimer’s disease are being so thoroughly researched.
If the human herpesvirus plays a significant role in the development of Alzheimer’s, surely then treatment procedures could be adapted to get the better of the disease? After all the use of established antiviral medications can be effective in treating virally triggered immune cascade reactions – a factor which can stimulate the growth of amyloid proteins and plaques.
If this is the case, could Alzheimer’s then possibly be prevented? Infection with the human herpesvirus would have to be a proven cause of the degenerative disease, and this is where current research questions are being focussed. Merely identifying a viral presence isn’t an answer in itself. A clue in this regard may lie in attaining a better understanding of the associated herpesviruses, and whether or not their presence in the brain is directly linked to the development of Alzheimer’s disease.
What are herpesviruses?
There are more than 100 known herpesvirus strains. Of these at least 8 infect human beings, many of which are actually quite common. (3) These include:
- Herpes simplex virus – type 1 (oral herpes)
- Herpes simplex virus - type 2 (genital herpes)
- Varicella-zoster virus (known to cause chickenpox and shingles)
- Epstein-Barr virus
- Cytomegalovirus
- Kaposi’s sarcoma-associated virus (also referred to as the human herpesvirus 8)
- Human herpesvirus 6 (A and B)
- Human herpesvirus 7
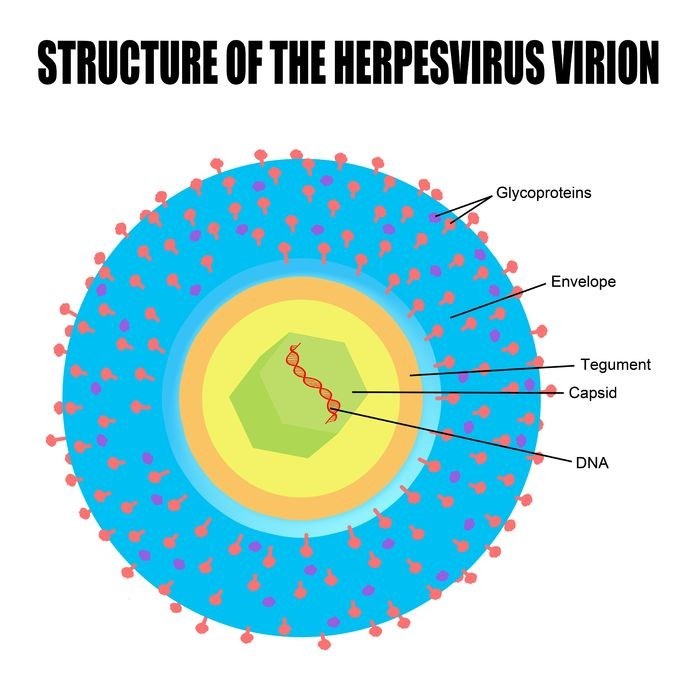
Herpesviruses are structured in unique layers which contain double-stranded DNA. The genes of these viruses replicate in a specific order, eventually allowing virus particles (virions) to be transported to cell membranes. As these released virus particles mature, the host’s (an infected person’s) cells perish.
The virus particles may also exist within the body in a latent state, making the virus inactive for a period of time. Symptomatic manifestations can arise causing illness (due to an active infection) and then become dormant for some time (i.e. months or even years). A latent herpes virus can reactivate at any particular time during a person’s lifetime. While this is an expected mechanism of a virus, the precise underlying reasons as to why this occurs are not very well understood. In the case of the herpes simplex viruses, certain stimuli do appear to trigger viral reactivations – these include stress, exposure to ultraviolet light and even menstruation – but such triggers aren’t well defined as a possible causal link.
Herpesviruses are classified into one of three sub-family groupings – alpha herpesviruses, beta herpesviruses and gamma herpesviruses. Human herpesviruses 6 and 7 are generally considered gamma herpesviruses, however they also display host range mechanisms (i.e. the ability to infect certain cells within the host) of beta herpesviruses. Gamma herpesviruses characteristically have a more limited host range.
Herpesvirus strain groupings which have a short replicative cycle (or reproductive cycle) include ‘A’, herpes simplex viruses (1 and 2) and the varicella-zoster virus. Cytomegalovirus, ‘B’ herpesvirus and human herpesvirus 6 and 7 tend to have a longer replicative cycle. Viruses with short cycles typically have a broad host range (i.e. the ability to infect and reproduce in a wide variety of organisms or cells), while longer cycles have a host range that is more restricted. Other host range herpesviruses that are restricted include the human herpesvirus 8 and Epstein-Barr virus.
Short cycles mean that virus particles are able to destroy host cells within hours. Longer cycles mean that this mechanism of infection takes place over a period of days.
Human herpesvirus 6 is more commonly associated with kidney transplant rejection, acute liver impairment or failure and infections affecting the central nervous system. Herpesvirus 6 is also associated with the development of roseola, a mild infection affecting young children resulting in a high fever and rash (also referred to as sixth disease).
Although the mode of transmission for herpesviruses 6 and 7 is not very well defined, it is agreed that most human beings appear to have a high prevalence of antibodies to these viruses very early on in life. (4) This suggests that the majority of human beings are thus exposed to these viruses at some stage during early life. Aspirated / inhaled viral secretions (also referred to as oropharyngeal secretions) are believed to be experienced in the home which may be one reason why antibodies are so high from a young age. Viral particles are transmitted via a host with a compromised immune system. This is yet to be sufficiently documented, however.
To date there is no official therapy for infections with herpesvirus 6 and 7. Most medical doctors who have detected these viruses in a patient may implement antiviral treatment (where necessary – i.e. where severe signs of infection occur) commonly used for cytomegalovirus since it is considered to be closely related, and thus the most effective agent to help clear an active virus.
In terms of prevention, there is only one licensed vaccine, and this is targeted to protect against the varicella-zoster virus in high-risk population categories.
How were herpesviruses identified in relation to Alzheimer’s disease?
The U.S. research team, with permission from the National Institutes of Health (who provided the donor samples and other data), initially set out to analyse post mortem brain tissue samples comparing those with diagnosed Alzheimer’s disease to others without the condition. The team analysed more than 600 post mortem brain tissue samples (from diagnosed Alzheimer’s disease patients) donated for research and more than 300 from non-dementia individuals (i.e. those with no neurological signs of brain disorder).
The team intended to assess 6 key regions within the brain, merely to see if there were any comparable differences, and if there were, to explore them further. The team assessed brain regions that experience changes resulting in early indications of Alzheimer’s disease, comparing these with those that develop more profound neuronal reductions later on. The purpose was to better understand the dramatic changes a patient experiences during progressed stages of the illness so as to help identify any possible ways treatment for the condition could be enhanced for both current and future patients.
“We were looking for genes that changed during the progression of Alzheimer’s disease, especially ones that changed most dramatically,” says Dr Samuel Gandy who is the associate director of the Mount Sinai Alzheimer's Disease Research Center, as well as a professor of neurology and psychiatry at the Icahn School of Medicine at Mount Sinai.
The team may not necessarily have expected to identify a viral connection, but this was the most significant difference that there was to be found. It’s not the first time, however, that herpesviruses have been linked with Alzheimer’s. Quite a few past studies have noted at least one of the human infecting varieties, and HHV 6 (A and B) has featured in some capacity or another too. (5) The herpes simplex viruses and cytomegalovirus varieties have featured more prominently in previous studies. Now HHV 6A and 7 have a spotlight cast over them.
The research team conducted a DNA (deoxyribonucleic acid) sequencing process and were able to determine detailed profiles of all inherited genes in the donor samples (whether diseased and not). The team then looked at RNA (ribonucleic acid) in order to identify and detail gene expression for each donor sample. From there, they made use of laser-captured neuronal gene expression data and clinical assessments for each sample of Alzheimer’s donors so as to best determine the nature of cognitive dysfunction during life. The team also made use of post mortem neuropathologic assessments in order to identify the severity range of amyloid protein tangles and plaques which are characteristic of the disease.
When looking at the identified genes of each participant, the team noted that human herpesviruses 6A (HHV 6A) and 7 featured fairly prominently. This viral presence may have a role in regulating the expression of DNA genes, including some of those considered high risk for Alzheimer’s disease and which are involved in the processing mechanisms of amyloid protein accumulation.
Viral levels appeared to be most prominent within the areas of the brain where Alzheimer’s disease is likely to have developed. These levels were not as high in non-disease sample donors. Human herpesviruses 6A and 7 appeared to be most prominent in the superior temporal gyrus (the primary auditory cortex which processes sounds and an area responsible for multisensory integration) and anterior prefrontal cortex (and area where complex planning and problem-solving functions take place) regions of the Alzheimer’s disease brain tissue samples.
"When you look at the viral genes in the setting of the network they're connected to, they often regulate the expression of genes we think are related to Alzheimer’s disease," Dr Gandy adds.
The levels of the virus detected in the brain tissue samples also appeared to correlate with the last recorded clinical dementia ratings (a score that is performed by a medical doctor quantifying the severity of a person’s symptoms) before death. These ratings detail both neurological evidence (obtained through testing) and behavioural symptoms noted during follow-up consultations. Those with higher viral DNA in the brain appeared to have more severe symptoms prior to their demise.
Thus, the research team began to question whether the development of Alzheimer’s disease could potentially be the result of the brain’s response to the presence of HHV 6A and 7 in the body. That said, they’re not jumping to conclusions and are certainly not concluding that herpesviruses are by any means a primary cause of Alzheimer’s disease development.
Higher viral DNA in the brain does, however, appear to have some role in the neuro-networks which directly influence the development of the disease. It therefore may be a contributing factor, either with regards to disease development or the progression rate, although this requires further and more detailed research. It’s possible that (potentially latent or reactivated) herpesviruses either activate genes that are in close proximity to those that influence Alzheimer’s development or suppress them.
Further research is needed to determine whether high levels of herpesviruses cause an immune or inflammatory reaction which could potentially spark Alzheimer’s development, or if their presence in the brain is more so as a result of the organ being unable to defend itself from an altogether different dysfunction. Whether herpesviruses are a direct cause or a secondary complication of sorts, theoretically antiviral treatment should be a viable option for treatment. It’s worth investigating further.
Clinical trials (small and larger population groups) using antiviral medications may very well be on the cards to determine if such treatment could inhibit the production of herpesvirus strains. Researchers can now assess the blood, spinal fluid and other bodily fluids of living diagnosed patients to determine the potential presence of herpesvirus strains. If these are present, testing and research analysis can be applied so as to ascertain whether a favourable treatment outcome may be possible.
Should further research be able to determine a more precise causative link, antiviral medications which are already fairly effective for treatment could then be very useful for affected Alzheimer’s patients. To what extent? Well, more analysis will need to be done in order to define this. If a direct causative link is not relevant however, what role could herpesviruses actually play then? Research will need to determine this too. There is still a human genetic and viral question mark lingering about for which a precise answer is not yet entirely clear.
For a disease with highly complex development and progression mechanisms, being able to determine anything specific is a major feat. If herpesviruses do indeed play a significant role in the development of Alzheimer’s, this team of researchers may just have opened a door to a potentially crucial breakthrough in medical science. It’s not entirely certain just what drives the long-term progression of brain changes or cognitive dysfunction, but such research has tapped into ‘a little something’ that surely paves a new path for further exploration with regards to precise causative mechanisms.
Certainly, the presence of viral DNA is significant, and in theory, should give way to potentially crucial advancements. The precise impact of these viruses can now be analysed further in future experimental studies. This will be helpful in determining implications as they apply to effective treatment, and even, possibly, prevention of the condition. This research doesn’t significantly shorten the path in this regard, but it does enable more specific details to be included in research conducted in future.
In a sense, herpesviruses may just be another puzzle piece. At this stage, there are more questions than answers and research will investigate the direct relation between herpesviruses and Alzheimer’s disease, whether causal or not – it could be that those prone to developing Alzheimer’s are actually just more susceptible to acquiring viruses.
With each new piece of information, a clearer picture of what causes Alzheimer’s disease is being built up. The ultimate answer could potentially lead to the kind of treatment advancements every specialist in this area of expertise, those affected by the condition and their loved ones would ultimately like to see.
Until then, we look forward to more research findings…
References:
1. Neuron. 21 June 2018. Multiscale Analysis of Independent Alzheimer’s Cohorts Finds Disruption of Molecular, Genetic, and Clinical Networks by Human Herpesvirus: https://www.cell.com/neuron/fulltext/S0896-6273(18)30421-5 [Accessed 26.06.2018]
2. World Health Organization. December 2017. Dementia Fact Sheet: http://www.who.int/news-room/fact-sheets/detail/dementia [Accessed 26.06.2018]
3. National Center for Biotechnology Information (NCBI). 1996. Medical Microbiology. 4th edition. Chapter 68 - Herpesviruses: https://www.ncbi.nlm.nih.gov/books/NBK8157/ [Accessed 26.06.2018]
4. US National Library of Medicine - National Institutes of Health. March 2002. Neutralizing Antibody Responses to Human Herpesviruses 6 and 7 Do Not Cross-React with Each Other, and Maternal Neutralizing Antibodies Contribute to Sequential Infection with These Viruses in Childhood: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC119946/ [Accessed 27.06.2018]
5. US National Library of Medicine - National Institutes of Health. July 2002.Herpesviruses in brain and Alzheimer's disease: https://www.ncbi.nlm.nih.gov/pubmed/12115887 [Accessed 26.06.2018]