It’s been almost a century since the discovery of penicillin and the development of various medication classes which directly treat and prevent infectious disease. Mass production (since World War II) and prescription use has gotten a handle on many routine infectious bacterial conditions, saving healthcare sectors significant amounts of money and, in many a case, the lives of the world’s populations.
Both the public and healthcare professionals, especially in the developed world, have grown accustomed to the availability of antibiotics. There are numerous antibiotic classes which doctors can prescribe when treating a patient’s condition. For quite some time, these medications have been effective treatment mechanisms. Without effective antibiotics available to medical personnel, there is a very real risk of illness that could potentially see a regression in healthcare successes and a spike in many bacterial infections that have, until now, been routinely controllable.
The headlines of the past year or so have focussed on ‘antibiotic resistance’ and specific conditions that are showing increasing levels of resistance to common first-line treatment practice such as gonorrhoea.
With bacteria constantly evolving, researchers are having to do a whole lot more than ‘tweak a molecule’ in existing antibiotics – as has been done in the past. A new class of antibiotics may now be required and as such, researchers have shifted gears and are proactively seeking viable solutions. But what does this actually mean? And what are researchers doing to try and prevent a potential healthcare crisis?
What does ‘antibiotics resistance’ actually mean?
Bacterial infections and the often devastating illness they can cause prompted the need for antibiotics to be developed in the first place. With their invention, the treatment of ill-health due to infection with bacterial organisms became far more manageable, and preventable too. And not just for human beings, but also for animals.
In fact, antibiotics are medications that medical health professionals have come to rely upon as a primary mode of treatment for bacterial infections in recent decades. Common infections can quickly be controlled and individuals with compromised immune systems can also benefit from using these drugs in a preventative capacity – for instance, antibiotics are routinely prescribed for patients undergoing surgical procedures to prevent infection.
With their use during the past century, sizable declines in mortality rates have been achieved. This is a massive feat for those in the medical industry and also why the development of these drugs has been of such vital importance. Instances of some infections and their related complications which were fairly common back in the day, have been dramatically reduced, so much so that occurrences of these in recent times have been rare, syphilis being one such example.
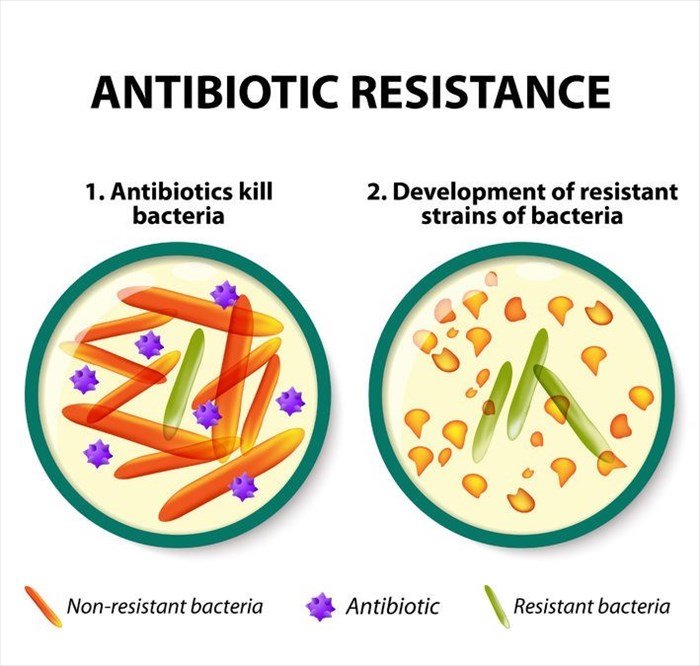
Alarmingly, a growing number of conditions that were once fairly easily treated with antibiotics are now becoming problematic, and quickly too. Bacterial organisms are slowly but surely gaining the upper hand over the drugs used to combat them. If it’s a war, bacterial organisms are the army that’s gaining momentum and ‘winning the battle’.
It is well known that bacterial organisms are quite adaptable – microorganisms are able to adapt to their host environments and evolve. Once they achieve this, they are able to multiply and thrive, developing resistance. They are then able to spread with more force and are thus better equipped to survive within their host (i.e. the body). In this ‘basic’ way, they become resistant to the medications formulated to kill off their proliferative capabilities and rid them from the body.
Growth of bacterial organisms leads to the development of new strains – another term making the headlines lately is ‘superbugs’. These are effectively newly developed bacterial strains that are increasingly resistant to antibiotic treatment – not just first-line options, but sometimes multiple drug classes too.
The more often a type of antibiotic is used to combat a specific bacterial organism, the more resistance has been observed and this inevitably compromises the effectiveness of the drug in question.
Some of the most notable challenges associated with antibiotic resistance
Global healthcare concerns aside, antibiotic resistance directly impacts food security, agriculture and the global economy too. With infections becoming increasingly difficult to treat with the antibiotics currently available, healthcare now faces a rather complex challenge. Coupled with repercussions like longer in-hospital care and increased medical costs, a re-emergence of infection treatment challenges that have long been under control could have significant impact on the global population once more.
The longer a person requires hospitalisation, the more at risk he/she becomes and the greater the challenge becomes for medical professionals to combat infection and return them to good health or even keep them alive. Poorer nations would surely feel the worst of the impact should no current antibiotics be effective at all.
A rise in mortality rates as a result of previously treatable infections becoming resistant to traditional methods of treatment has been seen recently. Thousands of drug-resistant conditions are being reported annually in respective regions and countries. Worst case scenario - as resistance continues to gain the upper hand, treatment options may become fewer and fewer, and the several hundred thousand annual deaths (as currently noted) could easily spike, reaching the millions, in the coming decades.
Overuse of antibiotics and an ever-increasing demand
The overuse of antibiotics is yet another news-grabbing headline with billions of doses currently being taken by human beings and animals. It is unfortunately a reality that many antibiotics are being misused for medical conditions that these medications are not designed to treat, like viruses. Others are being replicated and sold over-the-counter or even via the internet – without a prescription. No antibiotic should ever be made available without a valid prescription directly from a medical doctor. The impact is a sizable amount of revenue is lost by drug manufacturers and the repercussions involve growing drug resistance.
The Centers for Disease Control and Prevention (CDC) has said that they believe at least one third (30%) of antibiotic prescriptions are likely unnecessary and contributing to a worsening of the problem. (1)
The demand for antibiotics has spiked in recent years. Between the years 2000 and 2015, the estimated 39% consumption / usage rate increase (a figure obtained from the analysis of 76 countries) was assessed in a research study published in Proceedings of the National Academy of Sciences. To provide further context to this increase, that’s an estimated 21 to 35 billion additional daily doses being consumed which equates to a 65% increase in daily consumption. (2) Interestingly, low and middle-income nations such as in India, China and Brazil are driving this increase.
Usage increase in these countries is not entirely a bad thing as this means that poorer nations are gaining access to better healthcare. The problem may be that many of the conditions antibiotics are being prescribed for, are in fact preventable (i.e. infections can be prevented by other responsible means, like better hygiene practices).
The rapid need for the development of new generation antibiotics
With so many classes of antibiotics (antimicrobials) having entered the market over the years, the development of newer versions has stalled somewhat. Until the fairly recent past, the need for other newly developed and mass-produced drugs hasn’t been a primary requirement. For many researchers the focus has shifted to providing treatment options for incurable diseases and genetically complex conditions. Now, the need is changing somewhat, and the risks are being urgently reassessed by ‘those in-the-know’.
If the current antibiotics become ineffective due to resistant strains of bacteria, this means that we, the public, can easily catch a bacterial infection, like a UTI (urinary tract infection) or even strep throat and it may no longer be a treatable condition. Already red flags regarding the effective treatment of tuberculosis (TB) and sexually transmitted diseases, like gonorrhoea have surfaced. It’s also been established that ‘super-gonorrhoea’ is now very much a reality and antibiotics which would normally be used in its treatment are ineffective.
The rise of new drug-resistant bacterial strains
‘Nightmare bacteria’ is another term recently used to highlight antibiotic resistance. The CDC’s Antibiotic Resistance Lab Network (ARLN) recently noted findings of more than 220 germs that contain ‘unusual’ resistance genes in the USA. (3) Unusual types of resistance have the potential to quickly become problematic – hence why they’ve been labelled as ‘nightmare bacteria’. Along with rapid growth in the body, bacterial organisms that contain these genes are also adaptable in that they can share their resistance with other organisms, further hindering treatment effectiveness.
Aggressive responses (such as recommended containment strategies from the CDC (4)) to these unusual types offer the best strategy for cancelling out their potentially harmful mechanisms before they become common occurrences.
The WHO (World Health Organisation) has already published a list of globally recognised antibiotic-resistant bacteria (the ‘priority pathogens list’) (5) – the publication is aimed at assisting researchers across the world in developing new antibiotic classes for use now and in the near future.
International organisations are thus currently investing in research, with many analysts being of the opinion that a coordinated effort from countries from all corners of the globe sharing expertise is now needed if a crisis is to be averted. Antibiotic resistance is no longer a ‘future problem’ or isolated to just one region of the world, it’s very much a current global challenge with potential for serious repercussions that could affect members of the public as early as today.
Combatting bacteria in a new way
A research team at the University of Illinois at Chicago / UIC (USA), along with biotechnology company, Nosopharm (Lyon, France) have recently presented a new option to help combat the growing number of challenges now faced by medical professionals – a new class of antibiotics.
Nosopharm identified the antibiotic, which they named Odilorhabdins (ODLs), when they noted that its compounds appeared to have a very distinctive way of eliminating bacterial organisms. With their interest piqued, the team enrolled the help of UIC for further investigation.
The phenomenon that led them to this discovery was first identified in nematode worms which reside in soil, the team observed that insects are a colonised food source for them. The team screened at least 80 cultured strains of bacteria (produced by the nematode-symbiotic bacterium, Xenorhabdus / X. nematophila) that they identified as being carried by the worms.
This bacterium (and its microbial activity) was observed as being able to assist the worm in killing prey insects with the release of active compounds. These compounds (toxins and immune-modulators) were then isolated by the team. The chemical structures of these active compounds were engineered into derivatives (antibiotic secretions), which through observation, were noted as having the ability to halt bacterial growth by disrupting the interpretation of the bacterium’s genetic code. This suggested to the team that the antibiotic compound may offer a potential solution in their quest to fight antibiotic resistance.
Could ODLs help to combat resistance or infections that have now become difficult to treat? The study published on 5 April 2018 in Molecular Cell suggests that they can… (6)
ODLs are characterised as naturally produced antibiotics that target ribosomes – molecular (bacterial) cells which produce the proteins needed in order for bacteria to function. ODLs bind to the ribosomal subunit in a way that shows promising antibacterial capability and could serve as the next generation of treatment for bacterial infections. The binding of ribosomes is not unique, it is already present in the current clinically used antibiotics, however, the location (NOSO-95179) on the ribosome targeted by ODLs is.
Yurv Polikanov, assistant professor of biological sciences at the UIC College of Liberal Arts and Sciences, says that this unique binding site “has never been used by other known antibiotics."
Through in vivo and in vitro testing, the team determined that the disruption of the bacterium’s genetic code occurs at the stage where bacterial cells come into contact with ODLs. Interpretation of the ribosome is disturbed which results in molecular flaws (i.e. ‘miscoding’) during the protein production process. Miscoding then initiates the production of flawed proteins and halts bacterial growth. Not only that, it also causes bacterial cells to die off.
With the team’s interest further piqued , the Nosopharm researchers began a complex testing process, analysing the ODL compounds even further. Bacterial pathogens (Gram-negative and Gram-positive) that are currently high on the risk list and known to develop resistance to currently used drugs were analysed by the team using microbiological testing and mice. Some of these included:
- Escherichia coli (E. coli)
- Enterobacter aerogenes
- Enterobacter cloacae
- Enterococcus faecalis
- Klebsiella pneumoniae
- Proteus mirabilis
- Serratia marcescens
- Staphylococcus aureus
Findings noted that participating mice were able to be cured. A Gram-negative bacterium, Carbapenem-resistant Enterobacteriaceae (CRE) is one example which the research team noted as having the potential to be successfully eliminated. CRE currently are high on the list of difficult-to-treat bacterium and can cause conditions such as urinary tract infections (UTI’s), pneumonia and sepsis.
Inhibiting enzymes that bind to the decoding centre of the bacterial ribosomes and the resulting stimulated miscoding process has shown enough in animal models for researchers to conduct further analysis in clinical candidates (i.e. human beings).
The team believes that ODLs may very well be a new class of antibiotic which has very promising capabilities for treating resistant bacterial infections that are currently unresponsive to the first-line treatment drugs in circulation today. The therapeutic potential which this team observed through collaborative effort and combined expertise could mean that a new class of antibacterial agents are not that far from development phase, and this will surely provide both the medical industry and the public with some hope. We’ll keep you updated as further developments take place.
References:
1. Centers for Disease Control and Prevention. 3 May 2016. CDC: 1 in 3 antibiotic prescriptions unnecessary: https://www.cdc.gov/media/releases/2016/p0503-unnecessary-prescriptions.html [Accessed 09.04.2018]
2. Proceedings of the National Academy of Sciences of the United States of America. 26 March 2018. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015: http://www.pnas.org/content/early/2018/03/20/1717295115 [Accessed 09.04.2018]
3. Centers for Disease Control and Prevention. Antibiotic Resistance - Containing unusual resistance: https://www.cdc.gov/vitalsigns/containing-unusual-resistance/ [Accessed 09.04.2018]
4. Centers for Disease Control and Prevention. 27 Octover 2017. Interim Guidance for a Health Response to Contain Novel or Targeted Multidrug-resistant Organisms (MDROs): https://www.cdc.gov/hai/containment/guidelines.html [Accessed 09.04.2018]
5. World Health Organisation (WHO). Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics: http://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1 [Accessed 09.04.2018]
6. Molecular Cell. 5 April 2018. Odilorhabdins, Antibacterial Agents that Cause Miscoding by Binding at a New Ribosomal Site: http://www.cell.com/molecular-cell/fulltext/S1097-2765(18)30182-5 [Accessed 09.04.2018]