For those who suffer from this rare autosomal recessive condition (blood disorder), a ray of light has been shone for the future treatment of pain-related symptoms of sickle cell disease.
To have achieved a new means of treating a condition without a cure, for many in the medical field, the approval of a new medication that targets the (often debilitating) painful effects of the disease is a remarkable development.
For the physician, Dr Yutaka Niihara, who developed the medication, it’s been a long time coming too. For the past 25 years Dr Niihara has had an invested interest in developing what the FDA (U.S. Food and Drug Administration) has now approved, a drug known as Endari (an L-glutamine oral powder made by Emmaus Medical, in Torrance, California, USA). Now the medication is set to effectively treat the effects of pain in patients 5 years of age and older and promote a better quality of life. To date, the average life expectancy of a person suffering from sickle cell disease has been between 40 and 60 years of age.
What is sickle cell disease?
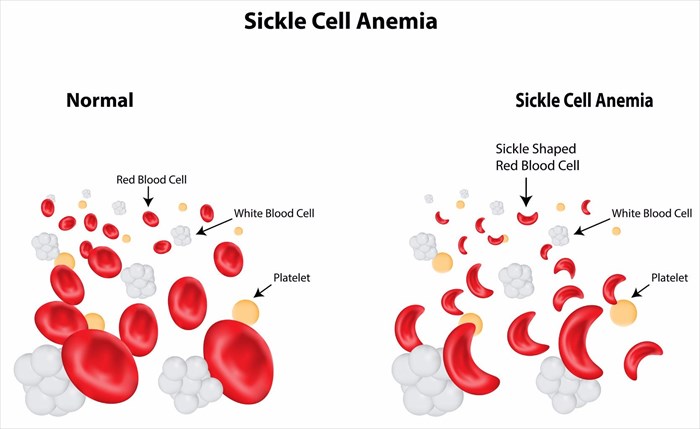
Sickle cell disease, or sickle cell anaemia is defined by an inherited grouping of red blood cell conditions or disorders. Two copies of non-sex chromosomes are genetically inherited (autosome genes) which compromise the normal capabilities of red blood cells (or RBCs). The effects are widespread, and influence virtually all bodily tissues. Red blood cells carry oxygen and without a sufficient amount being delivered throughout the body, an array of problems develop.
The normal shape of RBCs is likened to that of a disc which enables easier circulation and flexible movement in the body’s blood vessels (veins, capillaries and arteries). In sickle cell disease, the shape of some RBCs in the body are altered by the inherited autosome genes and become crescent-like instead of round. The abnormal shape (which is similar to that of a banana or crescent shaped moon) does not travel through blood vessels easily and thus the normal function of the cells is compromised or restricted.
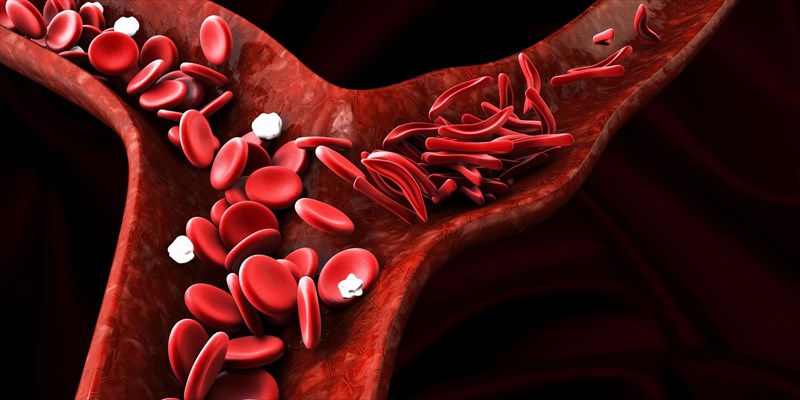
Crescent-shaped RBCs effectively become ‘sticky’ in the blood vessels and do not move with as much ease as the more rounded, normal cells. These abnormally shaped red blood cells are therefore more prone to becoming ‘stuck’ as blood circulates, thus restricting oxygen carrying blood to various portions of the body. Organs, muscles, bones and other tissues, including the skin, feed off this much-needed oxygen supply in order to carry out their specific functions for overall optimum health.
Without sufficient oxygen, damage to tissues takes place. One of the key symptoms that this results in is physical pain. ‘Sickle cell crisis’ is the term used to describe the periodic episodes of unexplained pain that occurs as a result of insufficient blood flow and oxygen. Restricted blood flow is especially worrisome when it comes to the smaller blood vessels in the body. This is predominantly where pain is most concentrated – the abdomen, bones, joints, skin and chest.
Intensity of pain can vary and last anywhere from a few hours to a handful of days. Frequency of crisis episodes also varies with some experiencing just a handful of painful occurrences in a year, and others a considerable amount more. Severe pain may even require hospitalisation and careful monitoring.
Chronic pain of this nature has further damaging effects. Areas most affected, such as the joints and bones, are at higher risk of further damage, and also impact surrounding tissues. Many sufferers of sickle cell disease develop ulcers as well . With pain comes swelling, often in the hands and feet, which can also be rather painful.
Other problems that develop include chronic bouts of anaemia (a shortage of red blood cells in the body due to sickle cells being destroyed quicker than the normal 120-day lifespan of normal RBCs), fatigue, lethargy, irritability, susceptibility to infections (a weakened ability to fend off infection such as the flu or pneumonia), problems with vision, and delayed growth rate (especially for infants, young children and teens).
To date, this disease has no potential cure and treatment merely involves daily management of symptoms so as to minimise or alleviate risk for a myriad of possible complications. These include ulcers, splenic sequestration (a very painful and sudden enlargement of the spleen), neurological problems (stroke or seizures), blindness, pulmonary hypertension (elevated blood pressure in the arteries of the lungs) and pulmonary fibrosis (scarring of the lungs), gallstones, abnormal heart rhythms and various other heart-related problems.
The path to developing Endari
Dr Niihara was training in haematology in 1989 when he first became familiar with sickle cell disease. Initially he had hopes of being able to specialise in cancer, but after seeing for himself the painful impact of sickle cell disease in various patients, his interests took on a different course. He shifted his focus and began researching all that he could about the disease and its effects.
Considered an ‘orphan disease’ (characterised as a condition that affects fewer than 200 000 individuals nationwide), sickle cell disease may be rarer than many cancers, but affects enough people in significant ways that Dr Niihara powered on with finding a means to alleviate such debilitating pain.
In early 2000, Dr Niihara took his many years of research to the FDA and began the process of motivating for what would become the now approved medication, Endari. The FDA granted Dr Niihara $1 million to proceed with a phase 2 clinical study for the drug.
This was excellent news, but it didn’t take long for a few speed bumps to hinder his research further. Dr Niihara had every intention of taking his research to an established pharmaceutical company in the hopes that they could help with the continuation of his clinical studies. Finding an interested company proved problematic for him, and so he developed Emmaus Life Sciences Inc. (a biopharmaceutical company focussed on the development and commercialisation of innovative treatments of rare / orphan diseases and disorders). For many pharmaceutical companies, developing medications for rare / orphan diseases comes at a sizable cost, which is considerably challenging when it comes to recouping expenses incurred in the process.
Now also the CEO of Emmaus, Dr Niihara continued his research for the development of the drug, and has successfully completed phase 2 and phase 3 clinical trials (completed in December 2013) with pleasing results.
The drug is not by any means a potential cure for sickle cell disease, but through trials, it has shown significant success in treating debilitating pain in particular.
What is Endari?
Endari, an orally administered pharmaceutical grade L-glutamine medication (in powder form), targets the body’s red blood cells. By administering glutamine (an amino acid that is commonly used in the biosynthesis / building of proteins), Dr Niihara has found that painful blockages in the bloodstream can be better alleviated, especially in the smaller blood-carrying vessels.
L-glutamine is already in natural abundance in the bloodstream, making up approximately 30 to 35% of existing amino acid nitrogen in the bloodstream. The substance has proven to have beneficial effects on the body’s digestive system, and helps to improve muscle growth, brain function, and blood sugar levels.
As an amino acid, glutamine is already in use for the treatment side-effects of chemotherapy – neuropathy (nerve pain), muscle and joint aches and pain, assists with protecting the immune and digestive systems, and improves recovery following bowel surgery or bone marrow transplants (due to its nitrogen compounds). Stomach ulcers, Crohn’s disease and ulcerative colitis have also shown symptom improvements treated with the substance. Glutamine is also already in use for treating certain symptoms of sickle cell disease.
Naturally, glutamine is produced in the body’s muscles and distributed in the bloodstream, promoting improved function in the organs that need it, as well as assisting other chemicals in the body to best serve their functions. Glutamine effectively helps to protect and restore tissues in the body. With a more targeted purpose, Dr Niihara has developed a drug (using the amino acid) that can potentially do a little more in helping to alleviate pain.
During the clinical trials, Endari was injected into RBCs and showed positive signs for the reduction of pain due to more flexible movement of sickle-shaped cells. Abnormally shaped cells became less ‘sticky’ and thereby resulted in less tissue damage. Improving circulation of red blood cells in the body can significantly treat some symptoms characteristic of the disease.
Endari given the ‘thumbs up’ by the FDA
FDA acting director of Haematology and Oncology products at the Centre for Drug Evaluation and Research, Dr Richard Pazdur announced the administration’s approval of the medication in July 2017, stating that it is now one of two approved drugs for severe and debilitating conditions.
Endari effectively becomes the first approved treatment medication for individuals living with sickle cell disease. Research spanning more than 20 years and proven successes in clinical trials using 230 participants between the ages of 5 and 58 (with sickle cell disease / anaemia and sickle β0-thalassemia , who have had at least two (or more) sickle cell crisis episodes within a 12-month period (before participation enrolment) have shown that the medication can provide significant symptom relief for this chronic / life-long condition.
Approval was based on a 48-week long clinical trial (followed by a 3 week-period of drug tapering) that noted a reduction in the need for hospitalisation where pain would normally be treated with injected anti-inflammatory medications (such as ketorolac) or a narcotic.
The randomised, double-blind, placebo-controlled clinical study divided participants into groups with one receiving Endari and another receiving a placebo. By week 48, the Endari group had required less hospitalisations (defined as an emergency room medical visit) than the placebo group, and those in the Endari group who were hospitalised, spent fewer cumulative days being treated for severe pain in hospital (an average of 6.5 days as opposed to an average of 11 in the placebo group).
A reduction in pain and severity also showed positive signs for lowering the risk of potentially life-threatening complications. Once such complication is acute chest syndrome, a leading cause of death in those with the disease, severely affecting functionality of the lungs. Other notable data showed a lowered risk of other complications such as splenic sequestration and priapism (prolonged erection of the penis without arousal). The Endari participant group showed a considerably lower risk of serious and life-threatening complications than those using the placebo (8.6% versus 23.1%).
Endari, like any other medication is not without side-effects and several adverse effects were found to occur in at least 10% of the participating patients. Only 2.7% of participating patients receiving Endari discontinued use due to adverse reactions (abdominal pain, a burning sensation, hot flash, hypersplenism / overactive spleen and dyspepsia / upset stomach and indigestion). Discontinuation resulted in at least one case each of a side-effect reaction.
Known side-effects of Endari include:
- Nausea
- Headache
- Abdominal pain
- Cough
- Constipation
- Pain in the chest, back and extremities
Based on the nature of positive data of the study, Endari is now an FDA approved oral medication (having received ‘orphan drug designation’) for the treatment of acute symptoms and complications of sickle cell disease in both paediatric (5 years of age and older) and adult patients.
The stamp of approval was also supported by the FDA Orphan Products Grants Program. Receiving an ‘Orphan Drug designation’ effectively provides encouragement for the development of other rare disease medications.
Development of the medication is assigned to Emmaus Life Sciences Inc. The recommended dosage of Endari is 10 to 30 grams (0.35 to 1.06 oz) to be taken orally, twice a day (dependant on a person’s body weight). The drug is available in powder form and must be mixed with 240ml (8 oz) of room temperature or cold liquid (water) or 113 to 170 grams (4 to 6 oz) of food before it can be safely ingested.
Future hopes for sickle cell disease treatment
With this stamp of approval, it is certainly raising hopes that federal regulators and more medication / drug producers will pick up the pace in developing better treatment measures for sickle cell disease (and other rare conditions), as well as better support extensive research.
For inherited disorders such as sickle cell disease which can be so debilitating, dramatically affecting the sufferer’s quality of life from the time of birth, research spanning decades is a significant amount of time to wait for improved treatment measures. That said, this newly approved medication may do more than elevate hopes, and successfully treat many with the condition.
There is still a lot about the condition that requires more resources and research. It is hoped that other research studies will be implemented sooner to specifically look at nutritional supplementation, and thereby establish sufficient recommended dietary allowances (RDAs) for those managing sickle cell disease.
Perhaps this newly approved medication will open a few more doors and achieve further successes in a shorter space of time. It certainly marks the end of a long drought in effective treatment for the disease. Dr Niihara is just one of many researching effective drug treatment alternatives, and it is already known that at least a dozen others are currently being studied. Many in the medical field hope that newly developed drugs without toxicity (for instance, carrying a risk of causing birth defects or cancerous conditions) are realistically possible.
On the agenda for Dr Niihara is the hope that Endari can be made an affordable medication. Normally, new medications cost a sizable amount of money, especially if being used for orphan disease management. Pricing of the drug has yet to be finalised, but it is hoped that costs can be kept well below current margins.
Dr Niihara also hopes that approval of the drug extends beyond the borders of the USA (such as in Europe and elsewhere), which will also help to improve pricing of the drug, making it available to more individuals managing sickle cell disease.