Treatment with activated charcoal in a clinical setting
In clinical settings, activated charcoal is FDA (U.S Food and Drug Administration) approved for use. The substance is technically not officially approved as a remedy or treatment for any popular alternative uses.
Activated charcoal is commonly implemented in a clinical emergency set up for the treatment of acute poisoning or overdose. If a patient presents with severe poisoning (high toxicity), more than one dosage may be required in order to counteract the absorption of toxic substances within the stomach.
In general, activated charcoal is considered an effective adsorbent for acute poisonings / overdose of substances with a molecular weight range between 100 and 1 000 daltons (a standard mass unit quantifying molecular mass).
Not every toxic chemical compound can be effectively treated with activated charcoal. If ingested, the following substances are not likely to be treatable with activated charcoal as it cannot prevent their absorption:
- Corrosive substances (e.g. alkalis)
- Acidic substances (e.g. boric acid, iron or lithium)
- Petroleum substances (e.g. coal oil, fuel oil, kerosene, cleaning fluids or paint thinners)
- Electrolytes (e.g. sodium, potassium or magnesium)
- Alcohols (ethanol)
Using activated charcoal in a medical setting to treat severe poisoning with substances which it is effective against, allows health professionals the best means to manage its effects. Many of the products in use today also contain a polyhydric alcohol (sweetener) which is added for its diuretic / laxative properties. This helps with removing it from the body via faeces / stool once the therapeutic process is complete. Products containing sorbitol should only be administered by a medical doctor so as to better control the potential side-effects which can include severe diarrhoea and / or vomiting.
Treatment procedures for poisoning and overdose
The absorption of toxic substances (also referred to as xenobiotics) or synthetic chemical agents within the stomach (or gastrointestinal tract) is aided by chemistry processes - hydrogen bonding, ionic forces (forces which hold ions together) and short-range electrostatic attractive forces occurring between unchanged molecules (these are known as van der Waals forces).
Adsorption typically is most effective when in direct contact with dissolved liquid phase substances in their non-ionised forms. Water-soluble molecules are not easily adsorbed by activated charcoal in the system. Non-polar (a chemical bond type whereby two atoms share a pair of electrons), inadequately water-soluble xenobiotics are better adsorbed by active charcoal.
Substances ingested in excess (i.e. an overdose) which can be successfully adsorbed by activated charcoal include:
- Acetaminophen (paracetamol)
- Aspirin
- Barbiturates (central nervous system depressants)
- Tricyclic antidepressants
- Theophylline (or 1,3-dimethylxanthine) – a drug used to treat respiratory diseases such as chronic obstructive pulmonary disease (COPD) and asthma
- Phenytoin (Dilantin) – an anti-seizure medication
Medical personnel will generally choose to administer activated charcoal when indications point to the presence of a toxic substance in the gastrointestinal (GI) tract. It must be determined before administration that the benefits of the adsorptive properties are likely to outweigh risks of use (i.e. potential side-effects).
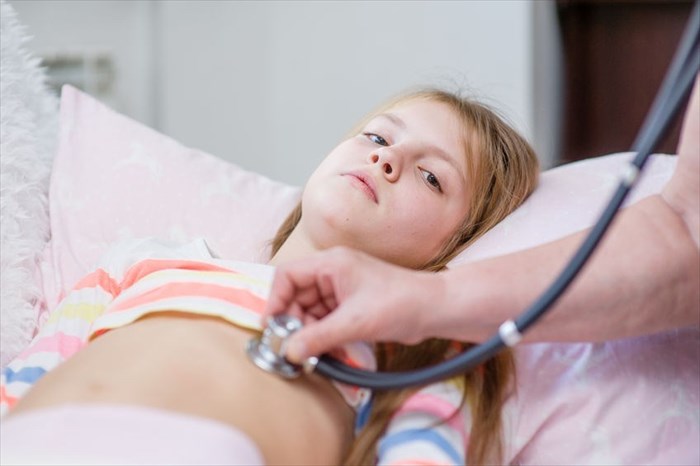
Medical personnel will likely check a patient for bowel sounds (also referred to as abdominal sounds) before administering an activated charcoal treatment. Bowel sounds are part of normal bodily function and are typically made when substances are moving through the hollow intestinal structures. These sounds echo through the abdominal area, signifying that the gastrointestinal tract is working. In the case of poisoning, the checking of bowel sounds may help to provide clues regarding the type of poisoning that has taken place and how it may be affecting the bowel.
To check bowel sounds, a medical professional will make use of a stethoscope, listening to the abdomen. The inability to hear any bowel sounds may indicate issues linked to a lack of intestinal activity, which may result in problems with gas and other substances (including fluids) causing blockages which could rupture the wall lining of the bowel. A decrease in sound volume, tone or frequency (hypoactive bowel sounds) may indicate slowed intestinal activity (which is typically only normal during sleep). Hypoactive bowel sounds, or a lack of sound may also indicate constipation.
Hyperactive (increased) bowel sounds or ‘tummy rumblings’ are sometimes audible even without the use of a stethoscope. Increased intestinal activity is common following the ingestion of a meal or when a person is experiencing diarrhoea. While listening to the bowel, a medical professional conducting the examination will likely query common symptoms like gas, nausea, vomiting and either the presence or absence of bowel movements as part of their physical check.
When ready to administer activated charcoal, intravenous (IV) antiemetics may also be given to lessen the likelihood of vomiting if this is anticipated in a patient.
In general, activated charcoal is given orally - to conscious and alert patients but capsules will not be used when treating acute poisoning. When administering treatment to a conscious patient, medical professionals will advise him / her to drink a prepared mixture slowly as rapidly consumed mixtures can often induce vomiting.
In some cases, activated charcoal may be administered via tubes (either via a nasogastric tube which is inserted through the nose and reaches the stomach or an orogastric tube that is inserted through the mouth, and reaches the stomach).
The palatability of activated charcoal has seen some improvement over the years of use. The substance is normally jet black in colour with a granular texture. Some formulations of the product are available as ready-to-use suspensions (doses of 15g, 25g and 50g) or pre-mixed with 25g of sorbitol (i.e. it is slightly sweetened).
If preferred, a semi-liquid mixture (or slurry) can be made with a ratio of 1:8 (1 part activated charcoal to 8 parts liquid such as water, flavoured syrup or carbonated soft drinks / colas) for adults and 1:3 for children. Mixtures may be given in opaque cups, covered with a lid, and accompanied by a straw if a patient finds the sight of the substance repulsive, making it difficult to swallow. Mixtures will be well shaken before being given to a patient.
At the time of treatment, especially in an emergency setting, a medical doctor may not know the precise dose of the toxic substance ingested. This makes the optimal dosing of active charcoal somewhat challenging. General treatment practice sometimes makes use of a 10:1 ratio (10 parts active charcoal to 1 part xenobiotic). If large dosage ingestions make calculating this ratio accurately a challenge (which can often occur), a medical doctor can opt for SDAC (Single Dose Activated Charcoal) administration (referred to as aqueous solutions). This can be calculated at 1g per kilogram of body weight. Alternatively, activated charcoal can be given according to recommended age dosage guidelines (1):
- Adults: 25g to 100 g
- Infants (younger than 1 year): 10g to 25g
- Young children (between the ages of 2 and 12 years): 25g to 50g
- Older children (12 years of age and up): 25g to 100g
If poisoning or toxicity levels are deemed severe or even life-threatening, MDAC (Multiple Dose Activated Charcoal) administration is recommended. At least 2 doses, given one after the other, are administered. It can happen that an additional (third) dose is required. Before administering treatment, some medical professionals may enlist the assistance of a regional toxicologist or local poison control centre to help determine the specific substance and the quantity ingested.
Multiple doses will be determined as necessary as a means to counteract ongoing xenobiotic absorption within the gastrointestinal tract which will be the case with the ingestion of high quantities of toxic substances.
Substances which may warrant Multiple Dose Activated Charcoal (MDAC) administration include:
- Amitriptyline (Elavil) – a drug used in the treatment of depression as well as ‘off-label’ for a variety of gastrointestinal and neuropathic pain related conditions.
- Amiodarone (Cordarone and Nexterone) – used in the treatment of irregular heartbeat (arrythmia)
- Carbamazepine (Tegretol) – used to treat epilepsy and neuropathic pain
- Dapsone or diaminodiphenyl sulfone (Aczone) – an antibiotic used to treat skin conditions
- Dextropropoxyphene – an opioid painkiller
- Digitoxin (Digitaline) – used to treat heart failure
- Digoxin (Lanoxin) – used to treat heart failure
- Disopyramide (Norpace) – used to treat irregular heartbeat
- Dosulepin (Prothiaden) – a tricyclic antidepressant used to treat depression
- Duloxetine (Cymbalta) - a selective serotonin and norepinephrine reuptake inhibitor antidepressant (SSNRI) used to treat depression, anxiety disorders, fibromyalgia and neuropathic pain.
- Lamotrigine (Lamictal) – an anticonvulsant used to treat epilepsy and bipolar disorder
- Nadolol (Corgard) – a non-selective beta blocker prescribed in the treatment of chest pain and high blood pressure
- Phenobarbital / phenobarbitone or phenobarb (Luminal) – an anti-epileptic medication
- Phenytoin (Dilantin) – an anti-seizure medication
- Piroxicam (Feldene) – a non-steroidal anti-inflammatory (NSAID) used to treat pain and inflammation
- Quinidine (Quinaglute and Quinidex) – prescribed to treat an irregular heartbeat
- Sotalol (Betapace) – used to treat abnormal heart rhythms
- Theophylline or 1,3-dimethylxanthine (Theolair and Slo-Bid) – used in the treatment of respiratory diseases
- Valproic acid (Convulex, Depakote, Epilim and Stavzor) – an anti-convulsant used to treat epilepsy, bipolar disorder and to help prevent migraines
- Verapamil (Calan and Isoptin) – used to treat high blood pressure
Activated charcoal dosages
Dosage MDAC administration for adults may be implemented as follows:
- Initial dose: 10.1 ratio (if possible, a medical doctor will try and tailor the dosage and any subsequent administrations according to the estimated level of toxic substance in the system). Alternatively, an initial dose may be given between 50g to 100g.
- Follow-up doses: Dosage ranges are administered at between 0.25g per kilogram to 0.5g per kilogram of body weight every 1 to 6 hours. Alternatively, follow-up doses can be given at between 25g to 50g every 4 hours.
Dosage MDAC administration for children may be implemented as follows:
- Initial dose: between 25g and 50g
- Follow-up doses: 10g and 25g every 4 hours
Pre-mixes with sorbitol for adults will only be given once (usually as the first dose) as this does carry of a risk of causing severe diarrhoea. Solutions containing sorbitol are not recommended for infants and young children at all due to the risk of diarrhoea. If necessary, a single administration of a sorbitol suspension can be given to teenage patients. Activated charcoal mixtures in water may be repeated until it is observed that clinical levels of toxicity have subsided and, if relevant, hyperactive bowel sounds have reduced. If repeat dosages are necessary, aqueous solutions (i.e. solutions containing water) should be administered.
Normally, treatment with activated charcoal is most effective when administered within 30 minutes to 1 hour of ingesting toxic substances. During the treatment period, medical personnel will continue to monitor bowel sounds, assess the person’s fluid and electrolyte status, and where relevant, the number of stools excreted or diarrhoea episodes encountered.
Note: Activated charcoal treatment for acute poisonings is not necessarily considered routine clinical practice but is an available approved option with beneficial results when administered within an hour of the ingestion of certain toxic substances. Some medical doctors may consider using activated charcoal even if ingestion is noted as having taken place longer than an hour before admission to hospital as it is reasonably agreeable that its ability to still offer some benefit cannot be entirely excluded. Administration of activated charcoal in the capacity of a home treatment for poisoning is not recommended. It is advisable to seek medical assistance as soon as possible if an overdose or poisoning is suspected.
Reference:
1. National Center for Biotechnology Information (NCBI). 23 January 2018. Activated Charcoal: https://www.ncbi.nlm.nih.gov/books/NBK482294/ [Accessed 25.04.2018]