- Chronic Traumatic Encephalopathy (CTE)
- Neuropathological stages of Chronic Traumatic Encephalopathy (CTE)
- Signs and symptoms of Chronic Traumatic Encephalopathy (CTE)
- How is Chronic Traumatic Encephalopathy (CTE) diagnosed?
- Is Chronic Traumatic Encephalopathy (CTE) treatable?
- How can Chronic Traumatic Encephalopathy (CTE) be prevented?
What is Chronic Traumatic Encephalopathy (CTE)?
Defining chronic traumatic encephalopathy and its potential causes
Currently considered a rare progressive neurodegenerative disorder (i.e. a condition that results in damage to nerve cells (neurons) which affects their function and / or causes them to die), chronic traumatic encephalopathy (also known as CTE) is believed to be the result of repetitive trauma to the head that has often taken place over an extended period of time.
Multiple traumas or injuries to the head are associated with delayed changes that occur in the brain, altering a person’s normal capacity to function. These changes influence how the affected person feels, thinks, behaves and / or moves. Neurodegenerative changes appear to occur gradually, presenting clinical symptoms years, or even several decades after a period of repetitive head trauma.
The first time this condition was written about in literature appears to be around the 1920s when abnormalities in the behaviour, cognitive (thought processing) and motor (movement) abilities of professional boxers were noted. Early literature characterised the condition as ‘punch drunk syndrome’ as a result. This term was later given a less derisive name - dementia pugilistica. Some literature also used the term ‘traumatic encephalopathy’ – which loosely refers to damage that has occurred in the brain. During the 1960s physicians began using the term ‘chronic traumatic encephalopathy’.
During the course of the next several decades, physicians appeared to realise that similar abnormalities could affect a much more diverse group, and not just professional boxers. Through the years, as more cases have surfaced, literature has highlighted the following as potential candidates in the development of the condition:
- Contact sports activities (such as American football (NFL), rugby, soccer, hockey and even professional wrestling)
- Military veterans exposed to combat activity (including injuries sustained following blast activity)
- Individuals subjected to domestic violence and abuse
- Those who engage in self-inflicted head-banging behaviour as a result of an underlying medical disorder or that which can occur during an epileptic seizure.
This raises questions with regards to just what type of injury to the head / brain ultimately causes CTE, and whether the degree of the impact and resulting damage play a role. The short answer is that both milder impact and extensive brain injury are currently associated with CTE development, but the extent and frequency of such cases are not yet well defined.
The following conditions may be contributing factors in the development of chronic traumatic encephalopathy:
- A traumatic brain injury (TBI) which occurs when altered brain function is identified (through diagnostic procedures) following a forceful impact to the head, resulting in either temporary or permanent abnormalities. The damage caused by an external force could be due to direct impact, the penetration of an object, blast waves or even something that encourages rapid movement of the brain inside the skull. Acute TBI can be a life-threatening event. A diagnosis of TBI is made according to the level of its severity, and may be classed as mild, moderate or severe – taking into careful consideration the period of time a person lost consciousness at the time of injury and where relevant, how long post-traumatic amnesia lasted. Long-term, traumatic brain injury can cause prolonged cognitive and physical disabilities, or post-concussion syndrome (PCS). It may also be a contributing factor to eventual chronic traumatic encephalopathy development.
- A concussion may be referred to as a mild traumatic brain injury (mTBI) which also results in cognitive, behavioural, physical and emotional changes during the initial days and weeks post-injury. Concussion is defined by transient brain function alteration, also as a result of external force. Persistent abnormalities can occur for up to several months. Symptoms of concussion include a persistent headache, coordination difficulties, confusion, disorientation, nausea, vomiting, dizziness, fatigue and sleep disturbances. The long-term effects of diagnosed mTBI is also clinically linked to CTE. Chronic symptoms involve cognitive, behavioural, mood and motor abnormalities similar to those attributed to CTE (which may be identified during analysis / diagnosis at a later stage).
- Repetitive sub-concussive events with subclinical effects (i.e. events that are asymptomatic), at this stage, cannot be discounted as possibly being involved in the development of CTE later in life either. ‘Sub-concussive head impact’ involves injurious contact, bumps or blows to the head which may not result in clinical symptoms, but can still trigger a cascade of neurodegenerative changes (i.e. changes to the way one functions as a result of the dysfunction and death of neurons (nerve cells) that subsequently affects the nervous system’s control of the body and communication between its parts). Thus, not all bumps and blows result in obvious symptoms at the time of impact but can still cause some degree of harm, especially if multiple traumatic events occur.
What appears to be a consistent factor in the development of chronic traumatic encephalopathy is repetitive impact. However mild, moderate or severe the impact may be, researchers and medical doctors cannot discount that such events do potentially raise the risk for neurodegenerative impairment later in life. The key is to find direct links.
An overview of injurious impact to the brain
While extensive or more severe head injury can result in obvious long-term complications, much is still being learned regarding sub-concussive impacts in relation to neurodegenerative damage (including the late / delayed onset of symptoms).
Sub-concussive versus concussive brain injury
Sub-concussive impacts are like micro-injuries with ‘wear and tear’ effects. Injury to the brain as a result may occur with less force than that which would result in a concussion. While a concussion shows obvious indications of impact (i.e. symptoms are noted in the person who sustains it), sub-concussive occurrences may not. The impact of a concussive blow to the head causes symptoms because, simply put, the brain experiences enough forceful injury to result in damage to its cells (neurons). The damage sustained means that these cells are not able to function as effectively as they did before the injurious impact.
On the other hand, clinically speaking, sub-concussive impacts do not typically result in symptoms as the cell damage sustained at the time of the blow is not severe, and as such is considered below the concussion threshold. However, it is important to note that a sub-concussive impact does not mean that no damage has occurred in the process. It merely means that any damage that does occur in the brain is not necessarily noticeable in the short-term.
Repetitive sub-concussive events
Should repetitive head / brain trauma occur over time, the impact thus results in an accumulation of damage which eventually causes enough weakening of the nerve cells (neurons) that obvious signs and symptoms may occur down the line. In other words, enough ‘wear and tear’ may result in damage down the line and compromised brain function.
An accumulation of sub-concussive impacts may contribute to deteriorations in memory and cognitive (thinking and thought processing) ability. The more impacts or blows a person experiences, the more impaired memory and attention may become. The strength of physical connections in the brain may also be affected by the repetitive strain sustained. Strain similar to that which can be caused by sub-concussive impacts can impair the interconnected ‘communication’ functionality of the brain and greater nervous system and inhibit the natural process of sending and receiving signals through these cellular ‘wires’ by depleting their structural strength.
Neuroimaging techniques, like functional magnetic resonance imaging (fMRI) have shown through study that alterations in brain function (or activity) and structural connection impairments can be linked to repetitive impact history. (1)
So, how are brain cells impacted by these types of brain injury?
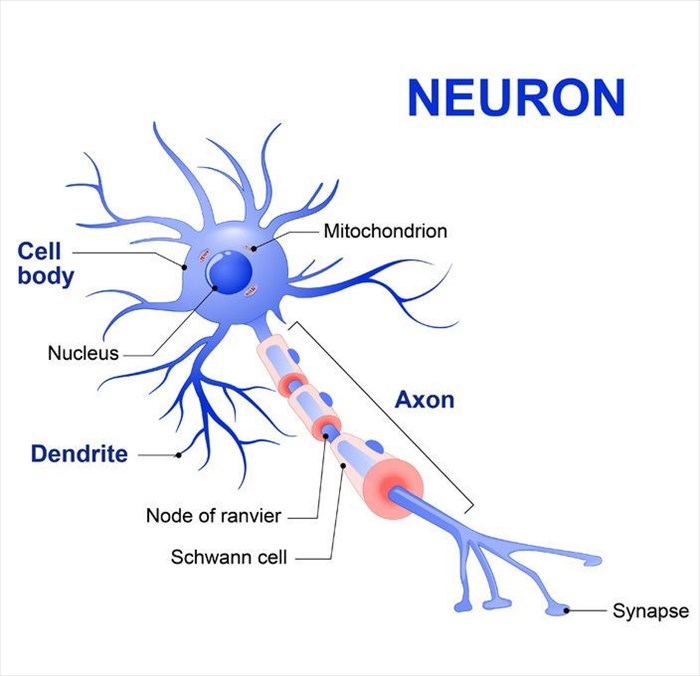
Each cell or nerve cell (neuron) consists of a cell body, nerve fibre (axon) and the endings of the axon (axon terminal). Electrical signals are transferred between nerve fibres and adjacent cells through the gaps between nerve cells (known as synapses) and this process is referred to as synaptic connection .
Nerve fibres (axons) are elongated and thin in shape, which effectively enables the communication activity being received and transmitted to reach other cells in different areas of the brain. The shape of nerve fibres is actually where abnormalities can occur as a result of impact to the head. Nerve fibres are considered somewhat fragile and the weakest point of each nerve cell (neuron), making these components more susceptible to injury if a blow or jolt to the head is sustained. As a result, damage that occurs due to head trauma typically affects these components of nerve cells more than the others.
Once damage to a nerve fibre occurs, the function of sending signals becomes impaired, causing a glitch in the system. The distribution of necessary chemicals and other materials required by cells (i.e. along the axon and at the axon terminal) is also hindered. Microtubules (which are much thinner structures then nerve fibres) run along the length of each nerve cell, including the nerve fibre and provide the ‘transportation system’ for these materials. This means that portions of each affected nerve cell are deprived of all the cell needs to function optimally. Damage to the microtubules along with the nerve fibre as a result of sub-concussive impact events may not cause these structures to break completely, but they can undergo increasing strain with repetitive injury occurrences.
With the microtubules being so thin and fragile, p-tau (phosphorylated Tau) is produced to support their structure by attaching to the outside of the tubes. Normally, the presence of this protein helps to maintain a healthy ‘transportation system’.
When a blow or jolt to the head weakens the axons and microtubule structures, the protein begins to disperse – detaching from the outside of the tube structures (this is known as disassociation). As microtubules break down, p-tau can enter the inside of the affected brain cells, sometimes altering their shape – this process is referred to as phosphorylation. The result is the formation of protein clumps within the brain cells.
Clumps of p-tau tend to enlarge, accumulating more of the protein which may then move to other areas of the brain as the disease progresses over time. This process is referred to as ‘prion spread’ and takes place gradually.
Even if multiple sub-concussive impact events do not occur during a person’s lifetime, these clumps can still accumulate more of the protein over time. The gradual process may be one reason why symptoms take such a long time to show up. Eventually, the slow clump accumulation and gradual movement affect enough brain tissues to result in functional alteration that is clinically noticeable (i.e. causing noticeable signs and symptoms).
What do doctors know about the potential causes of chronic traumatic encephalopathy (CTE)?
The degree of impact, number of blows and resulting injury in relation to the development of chronic traumatic encephalopathy is still something that is being intensively researched at present. There are still no clear answers regarding the precise causes and mechanisms of progression of this condition.
For the time being, while research is ongoing, the exact causes of CTE are loosely described based on a history of ‘sub-concussive head impact’ and symptomatic injury lines and what is known to occur in the brain when damage as a result of these events occurs. This appears to be the common denominator for now. Researchers appear to be in agreement that the longer a person is exposed to repeated blows or injurious contact (often over a period of several years), the more likely the chance that CTE may develop.
There are other factors to take into consideration, some of which include:
`1. P-tau
CTE is characterised by the presence of the protein, phosphorylated tau (also known as p-tau) which is deposited as clumps in the brain once damage has occurred. Identifying the presence of this protein, which is observed as neurofibrillary tangles or NFTs (twisted tangles of hyperphosphorylated p-tau fibres found in the brain cells), typically takes place neuropathologically – i.e. by studying tissue samples extracted from the nervous system either through surgical biopsy or as part of an autopsy (i.e. the examination of a body once someone dies).
Deposits of p-tau appear to eventually occur in a widespread pattern affecting the spaces around the blood vessels, within the depths of the fissures or grooves (sulci) in the cerebral cortex, around or beneath the innermost layer of the meninges (membranes that cover the brain and spinal cord) or the composite structure of the hypothalamus (a small region of the brain that contains small nuclei with a variety of functions including the regulation of certain metabolic processes as well as hormone secretion).
A build-up of this protein is what is believed to trigger progressive degeneration in the brain. Degenerative changes may begin to take place within as little as a few months or years, and even decades following injurious impact to the brain. Signs of degeneration become evident when problems like the following develop:
- Memory loss,
- Judgement impairment
- Confusion
- Depression (sometimes linked with suicidal tendencies)
- Aggression
- Irritability
- Difficulties with impulse control
Parkinsonism (an umbrella term for a group of neurological disorders that cause issue with movement similar to those characteristic of Parkinson’s disease like tremors, stiffness and delayed movement) and even the progressive development of dementia are also linked with advanced CTE.
The accumulation of p-tau is not entirely unique to confirmed CTE patients. It is also something that is seen in individuals diagnosed with Alzheimer’s disease, as well as in neuro-inflammatory conditions like multiple sclerosis (MS). The accumulation of the protein is, however, common in all cases where CTE has been officially diagnosed (usually only after death during an autopsy).
2. Post-mortem neuropathologic study (autopsy)
Actual diagnosed CTE cases have not occurred while the affected person is still alive. To date, CTE and the macroscopic and histopathological analysis of p-tau has only been determined during autopsy procedures. Brain tissue analysis after the demise of affected individuals and clinical interviews conducted retrospectively with living individuals who were close to a sufferer have sometimes led to an official diagnosis of chronic traumatic encephalopathy (CTE). Some analysed tissues have led to the diagnosis of other neurodegenerative conditions.
The largest tissue repository with a focus on CTE and other traumatic brain injuries is facilitated by medical professionals and researchers at Boston University who run the VA-BU-CLF Brain Bank. (2) The BU CTE Center is run in collaboration with the VA Boston Healthcare System, and together, teams of researchers are actively studying hundreds of donated brains and other central nervous system tissues, like spinal cords and eyes, in order to better understand the nature of CTE.
As researchers learn more through study, the clearer the signs and symptoms of CTE during life can become. As many clues and indications as there may be, there are still many gaps and questions yet to be addressed and answered. There is also a great deal to be done to determine the exact clinical course of the disease and how to identify its presence and progression during life.
As such, medical research is still working on determining exactly how the condition develops, and why it affects some individuals and not others. Research to date has identified many discrepancies and question marks. It’s not just certain sportsmen and military veterans exposed to head impacts that can develop CTE. A person may develop CTE even without a history of serious brain injury or concussions where symptoms were addressed and treated at the time. It is also possible that multiple blows (without clinical symptoms being evident thereafter) or serious injury may not result in CTE at all.
Thus, there is still a great deal regarding the pathobiological mechanisms associated with the development of CTE that is yet to be clearly understood. Once this is better identified and understood, diagnostic tools and treatment procedures can be developed for use while a person with the condition is still alive.
3. The role of genetics
Underlying factors in the development of CTE which are linked to possible genetic influences are not entirely well understood, as yet. What medical professionals do know is that not everyone that has been posthumously diagnosed with CTE had a history of repetitive head / brain injury – i.e. not every confirmed case of CTE has been from an individual who had a career in contact sports or serving in the military. Similarly, not all professional athletes involved in contact sport careers go on to develop neurodegenerative conditions either. This has led scientists to wonder if perhaps some individuals have some form of genetic susceptibility that contributes to the development of the condition.
One of the better studied genes in relation to neurotrauma (injury to the nervous system) is apolipoprotein E ε4 (APOE ε4), which is carried on chromosome 19. Apolipoprotein E (APOE) is produced in the central nervous system and is synthesised by astrocytes (star-shaped glial cells) and microglia (glial / immune cells of the central nervous system), as well as nerve cells (neurons) in the brain that are affected by some form of stress or strain. APOE normally functions by aiding nerve cell growth and maintenance, promoting repair and helping to mediate inflammatory responses in the brain.
**MyMed Memo: Glial cells are the most abundant cells in the central nervous system (CNS), they surround the nerve cells, supporting and insulating them.
APOE exists in several forms (or variant alleles) including APOE ε4 - APOE ε2 and APOE ε3 are the other variant forms. It is known that a high percentage of individuals with Alzheimer’s disease (nearly half) carry the APOE ε4 gene, making it a genetic risk factor. (3) It is also a risk factor in the development of dementia.
This has prompted researchers to look into a possible association between this gene and the development of chronic traumatic encephalopathy – to see if there is some potential explanation to be found as to why some individuals go on to develop the condition, but others do not. Studies have been able to determine that the presence of the APOE ε4 gene in living individuals can influence their cognitive function. Brain tissue analysis has also identified the gene in individuals with a subsequent CTE diagnosis, suggesting that it could be a possible risk factor, particularly where multiple impacts (repetitive trauma or injury) have occurred. (4)
An aggregate of tau genes is another area of focus whereby researchers are looking to see if a certain type of genetic profile may play a role. An aggregate tau risk gene score is something that is being assessed along with APOE ε4 in order to identify any potential predisposing factors in this regard that may make an individual more susceptible to developing CTE down the line.
The transactive response DNA binding protein 43 kDa (or TDP-43) is another noticeable factor in the diagnostic analysis of CTE. The transactive response DNA binding protein gene essentially provides instructions for the making of TDP-43 (a type of protein) (5) which is located within the nucleus of cells. The TDP-43 protein helps to control the production of the various different versions of other proteins (mostly in the nervous system) and influences cell function through the regulation process.
Found in most tissues in the body, TDP-43 attaches to DNA and facilitates regulation activity (known as transcription). This process is essentially one of several steps required in the production process of proteins. The protein also binds to RNA, facilitating stability, and has a role to play in the processing of molecules known as mRNA (messenger RNA).
Accumulations of malformed proteins as well as elevated blood levels of TDP-43 have been found to occur in many diagnosed CTE cases. Such elevations (or mutations) are also linked with other neurodegenerative conditions including Alzheimer’s disease and ALS (amyotrophic lateral sclerosis). Transactive response DNA binding protein genes may also have a role in causing frontotemporal dementia (FTD) without any indications of ALS.
When it comes to the development of CTE, the neurological underpinnings associated with genetic influence are not a highly understood area as yet. Studies have been done, some with mixed results, but many having been designed differently which may account for the discrepancies. Further research is needed to really consolidate findings and pin down a definite causal link so that the course of clinical changes in the brain can be better understood. This understanding may establish what kind of role genetics could play in the risk factors for CTE and to what extent permanent damage to the brain may occur. For now, genetic markers are still under intensive research in this field.
Who is most at risk for developing chronic traumatic encephalopathy (CTE)
Details surrounding the incidence and prevalence of chronic traumatic encephalopathy (CTE) are lacking, who and how many people are affected is thus not entirely known (at this point in time).
Experiencing some form of head impact does appear to be the most common link in the development of the condition. The nature of impact, however, certainly requires more research. Thus, the type of injurious blows and number of harmful events that may ultimately lead to CTE have not yet been precisely determined. Risk factors beyond head injury are lacking in broader understanding too.
There are other potential risk factors on the table and research is also yet to determine to what extent they may (or may not) contribute to the development of the disease. For now, there is no clear correlation with causality until this area itself is precisely defined.
Some proposed areas involved in research include:
- Total number of injurious impacts (including concussions or other traumatic brain injuries (TBIs) and sub-concussive impact events), type of trauma and frequency
- The involvement of substance use / abuse
- Medical comorbidities (like Lewy body disease, Alzheimer’s disease, motor neuron disease and frontotemporal lobar degeneration), including those of a psychological or psychiatric nature
- Associated inflammatory symptoms from certain medical comorbidities (these may relate to existing conditions like hypertension / high blood pressure, diabetes or even obesity)
- The role of genetic influences
- The potential role of gender – Do hormonal differences have any influence? Are neck circumference and muscle strength potential factors for consideration?
In terms of risk, a few serious injuries or concussions may lead to CTE later in life, as could many sub-concussive impacts over a period of time where no loss of consciousness takes place. By the same token, a history of recurring concussions (or even just one) may not ever result in CTE for some individuals. In the case of a boxer for instance, the number of rounds during the span of a career could be just as much a risk factor as the occasions the athlete was knocked unconscious.
Determining an exact pattern of risk is a vague area for the time being. It has not been clearly identified if the type of player positions in team contact sports has a role to play either – i.e. if one type of position (like offensive or defensive positions) is riskier than others due to the specific requirements of the game.
All researchers and physicians have to go on thus far is the one common denominator determined in their research and diagnostic analyses – and that is repetitive head trauma (whether symptoms are present or not). Every diagnosis made has this factor in common.
So far those identified as most at risk for CTE development (through actual diagnosed cases) have been:
1. Contact sport athletes (involved in professional games and practice sessions):
- Boxers – who suffer multiple blows or punches to the head.
- American football players – who experience multiple contact / impact events even when a helmet is worn over the head.
- Football or soccer players – who are exposed to multiple collisions with other players or headers (deliberately using the head to make contact with the ball).
- Hockey players / ice hockey players – who play in such a way that multiple contacts are made during defensive techniques such as ‘checking’ (intentionally using the body to apply pressure on an opponent in order to gain advantage of the puck) or even fighting during the game.
- Rugby players – who experience multiple contacts involving the head during tactical game playing.
- Professional wrestlers or martial artists – who sustain injurious contact that is made during the activity on multiple occasions.
Other sports may also be considered a potential risk for serious injury or sub-concussive impacts (on multiple occasions), like baseball, cricket or even basketball, where contact with an element of the game can result in harm to the head and brain. The aforementioned sports have, however, been implicated in the development of the condition specifically through actual post-mortem CTE diagnosis. It is possible that other sports where multiple injuries occur could result in the development of CTE.
2. Military veterans
- Injurious effects following exposure to combat and blasts.
3. Individuals subjected to physical abuse (domestic violence)
- Violent injury that repetitively occurs between a couple in an abusive relationship.
- Children and adults subjected to repetitive domestic physical abuse (within the home or elsewhere).
4. Developmental disorders associated with head banging behaviours, such as:
- Autism
- Rhythmic movement disorder (RMD)
- Stereotypic movement disorder (SMD)
Other influencing factors which may increase the risk of development of chronic traumatic encephalopathy and form part of ongoing research include:
- Age of exposure – young individuals, like children, exposed to contact sports may be at higher risk of developing CTE, especially if they are younger than the age of 12. Exposure to activities involving potentially repetitive head impacts during a vital neurodevelopmental period may play a role in elevating the risk for later life impairments linked with CTE. (6)
- Length of head impact exposure – individuals with longer contact sport careers may be at greater risk of CTE development than those who had shorter careers. Further research is needed to specifically pinpoint this as a definite risk factor however, as some former players have developed severe pathology associated with CTE and others that of a milder nature or not at all.
- Lifestyle comorbidities – with contact sports professionals and military veterans being the predominantly studied groups of individuals associated with diagnosed cases of CTE, comorbid factors (i.e. factors that may point to the presence of CTE as well as another disease that caused the issues experienced by the sufferer) have cropped up as potentially playing a role. Many of the subjects studied were found to have participated in substance use or abuse, like alcohol or recreational drug and even steroids or performance enhancing drugs usage. Further research is required to look into differentiating between the types of personality, behavioural and neuropsychiatric changes that can occur as a result of CTE or substance usage both independently and in combination. Currently, the extent to which substance usage may influence the clinical manifestations of CTE in affected individuals, or if at all, is not clearly defined. Many other diagnosed instances did not show a history of substance use, so such factors are not thought to function as causative agents at this stage but cannot be entirely discounted either.
References:
1. U.S. National Library of Medicine - National Institutes of Health. March 2017. The effect of repetitive subconcussive collisions on brain integrity in collegiate football players over a single football season: A multi-modal neuroimaging study: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5377433/ [Accessed 10.05.2018]
2. Boston University Research CTE Center. Brain Donation: https://www.bu.edu/cte/brain-donation-registry/ [Accessed 10.05.2018]
3. U.S. National Library of Medicine - National Institutes of Health. March 2008. Apolipoprotein E ε4 magnifies lifestyle risks for dementia: a population-based study: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3828889/ [Accessed 10.05.2018]
4. U.S. National Library of Medicine - National Institutes of Health. 2016. Translational Research in Traumatic Brain Injury - Chapter 9: Genetic Influences in Traumatic Brain Injury: https://www.ncbi.nlm.nih.gov/books/NBK326717/ [Accessed 10.05.2018]
5. U.S. National Library of Medicine - Genetics (Home Reference). March 2016. TARDBP gene - TAR DNA binding protein: https://ghr.nlm.nih.gov/gene/TARDBP [Accessed 10.05.2018]
6. U.S. National Library of Medicine - National Institutes of Health. March 2015. Age of first exposure to football and later-life cognitive impairment in former NFL players: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4371403/ [Accessed 10.05.2018]
Other Articles of Interest

Alzheimer's Disease
Do you find you or a loved one is suffering from progressive memory and mental deterioration loss in old age? It could be from Alzheimer's Disease...

Parkinson’s disease
Parkinson's disease is a progressive neurological disorder that gradually causes a degeneration of the brain's nerve cells responsible for the control and regulation of body movements. Learn more...
Amyotrophic Lateral Sclerosis (ALS)
ALS is a difficult neurological disease that affects the functioning of the nerve cells. Our article has all you need to know about ALS and coping with it.